A Comprehensive Guide to the Dynamic Nature of Chemical Reactions

Unit 8 in AP Chemistry delves into the fundamental principles of chemical equilibrium, providing a framework for understanding the dynamic interplays between reactants and products in reversible reactions. This unit is essential for developing the analytical and problem-solving skills necessary for comprehending a wide range of chemical processes.
The Basics of Chemical Equilibrium
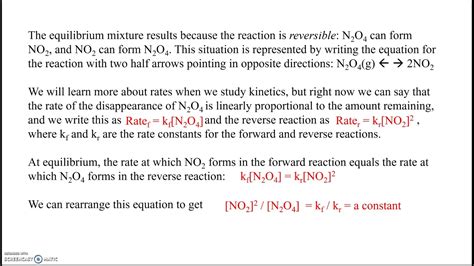
Chemical equilibrium represents the state in which the forward and reverse reactions of a chemical system occur at equal rates. This results in a constant concentration of reactants and products, despite the ongoing interconversions between these species.
The equilibrium constant (K) is a quantitative expression of the relative concentrations of reactants and products at equilibrium. For a general reversible reaction:
aA + bB <=> cC + dD
the equilibrium constant is given by:
K = [C]^c[D]^d / [A]^a[B]^b
where [A], [B], [C], and [D] represent the molar concentrations of the respective species.
Factors Affecting Chemical Equilibrium
Several factors can shift the equilibrium position of a reaction:
- Concentration: Increasing the concentration of reactants favors the forward reaction, while increasing the concentration of products favors the reverse reaction.
- Temperature: Raising the temperature shifts the equilibrium towards the endothermic reaction (absorbing heat).
- Pressure: For gas-phase reactions, increasing the pressure shifts the equilibrium towards the side with fewer moles of gas.
- Addition of Catalysts: Catalysts accelerate the rate of both forward and reverse reactions without affecting the equilibrium position.
Applications of Chemical Equilibrium
The concept of chemical equilibrium has numerous applications across various scientific fields:
- Predicting Reaction Yields: By calculating the equilibrium constant, chemists can estimate the maximum yield of a reaction under specific conditions.
- Buffer Solutions: Buffers maintain a relatively constant pH by utilizing a weak acid and its conjugate base, which act as a reservoir for H+ ions.
- Gas-Liquid Equilibria: The equilibrium constant for a gas dissolving in a liquid determines the solubility of the gas in that liquid.
- Heterogeneous Equilibria: These occur between species in different phases, such as solids and gases or liquids and gases.
Tips and Tricks for Mastering Unit 8
- Understand the Concept of K: The equilibrium constant is the foundation of all equilibrium calculations.
- Apply Le Chatelier’s Principle: Predict shifts in equilibrium position based on changes in concentration, temperature, pressure, and the presence of catalysts.
- Use ICE Tables: Construct Initial, Change, and Equilibrium tables to track the changes in concentrations of reactants and products.
- Master Equilibrium Calculations: Practice problems involving equilibrium constant calculations, calculating reaction yields, and analyzing equilibrium shifts.
FAQs
1. What is the difference between a closed system and an open system in equilibrium?
A closed system is one where no mass can enter or leave, while an open system allows for mass transfer. Equilibrium in a closed system is characterized by constant concentrations, while equilibrium in an open system may involve continuous inflow and outflow of reactants and products.
2. How can I determine the equilibrium position of a reaction using K?
If K > 1, the reaction proceeds more towards the products. If K < 1, the reaction proceeds more towards the reactants.
3. What is the role of catalysts in chemical equilibrium?
Catalysts lower the activation energy of both forward and reverse reactions, increasing the rate of attainment of equilibrium. They do not alter the equilibrium constant.
4. How does temperature affect the equilibrium constant of an exothermic reaction?
For exothermic reactions (heat is released), increasing temperature shifts the equilibrium towards the reactants (endothermic reaction).
5. How can I predict the solubility of a gas in a liquid?
The equilibrium constant for the dissolution of a gas in a liquid provides information about the gas’s solubility. Lower K values indicate lower solubility.
6. What is the significance of buffers in biological systems?
Buffers minimize pH changes in bodily fluids by absorbing or releasing protons in response to acid or base additions, maintaining a stable internal environment.