Introduction
Acids and bases are fundamental concepts in chemistry, playing crucial roles in numerous natural and industrial processes. Understanding these concepts is essential for students preparing for the AP Chemistry Exam and pursuing further education in science and engineering.

Acid-Base Definitions
Arrhenius Theory
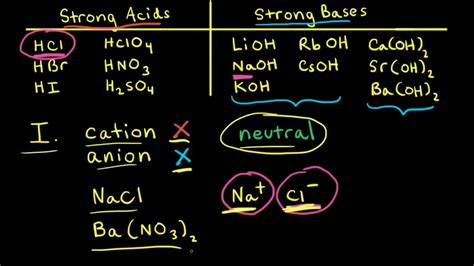
According to the Arrhenius theory, acids are substances that dissociate in water to produce hydrogen ions (H+), while bases are substances that dissociate in water to produce hydroxide ions (OH-).
Brønsted-Lowry Theory
The Brønsted-Lowry theory defines acids as proton donors and bases as proton acceptors. In this theory, acids donate a proton to form a conjugate base, while bases accept a proton to form a conjugate acid.
Acid-Base Properties
Strength of Acids and Bases
Acids and bases are characterized by their strength, which is measured by their respective ionization constants. The stronger the acid, the higher its ionization constant (Ka), and the more easily it dissociates to produce H+ ions. Similarly, the stronger the base, the higher its ionization constant (Kb), and the more easily it dissociates to produce OH- ions.
Table 1: Common Acids and Bases and Their Ionization Constants
| Acid | Ka | Base | Kb |
|---|---|---|---|
| Hydrochloric acid (HCl) | 10^7 | Sodium hydroxide (NaOH) | 10^-14 |
| Sulfuric acid (H2SO4) | 10^-3 | Potassium hydroxide (KOH) | 10^-14 |
| Acetic acid (CH3COOH) | 1.8 x 10^-5 | Ammonia (NH3) | 1.8 x 10^-5 |
pH and pOH
The pH of a solution measures its acidity or alkalinity on a scale from 0 to 14. A pH below 7 indicates an acidic solution, a pH of 7 indicates a neutral solution, and a pH above 7 indicates a basic solution.
The pOH is the negative logarithm of the hydroxide ion concentration and is related to the pH by the following equation:
pH + pOH = 14
Acid-Base Reactions
Neutralization Reactions
Neutralization reactions occur when an acid and a base react in stoichiometric proportions, resulting in the formation of a salt and water.
Example:
HCl + NaOH → NaCl + H2O
Titration
Titration is a technique used to determine the concentration of an unknown acid or base by reacting it with a known concentration of the opposite species. The equivalence point is reached when the moles of acid and base are equal, and the solution is neutral.
Acid-Base Equilibria
In aqueous solutions, acids and bases undergo equilibria in which the forward (dissociation) and reverse (association) reactions occur simultaneously. The equilibrium constant (Keq) is a measure of the extent of the dissociation reaction and is given by the following equation:
Keq = [H+][A-] / [HA]
where [H+] is the concentration of hydrogen ions, [A-] is the concentration of conjugate base ions, and [HA] is the concentration of the undissociated acid.
Applications of Acids and Bases
Acids and bases have numerous applications in everyday life and in various industries:
Industrial Applications
- Production of fertilizers and chemicals
- Refining of petroleum
- Manufacturing of plastics and textiles
Household Applications
- Batteries
- Cleaning products
- Food preservation
Biological Applications
- pH regulation in living organisms
- Digestion
- Enzyme activity
Common Errors
Misunderstanding the Arrhenius and Brønsted-Lowry Theories
Students often confuse the two definitions of acids and bases and fail to recognize that both theories provide complementary perspectives on these compounds.
Ignoring the Effects of Concentration on pH
The pH of a solution is not solely determined by the strength of the acid or base but also by its concentration.
Incorrectly Identifying Acid-Base Equilibria
Some students mistakenly believe that acid-base equilibria are always complete reactions, when in reality they are dynamic equilibria with both forward and reverse reactions occurring.
Tips and Tricks
Understand the Dissociation of Conjugate Species
For a strong acid, the conjugate base is very weak, and for a strong base, the conjugate acid is very weak. This concept helps in predicting the extent of dissociation in acid-base reactions.
Utilize the pKa Scale
The pKa scale provides a convenient way to compare the strength of acids. A lower pKa value indicates a stronger acid.
Practice Titration Calculations
Solving titration problems can strengthen your understanding of acid-base stoichiometry and equilibrium.
Future Applications
One promising area of research in the field of acids and bases is the development of biomimetic acids, which mimic the properties of enzymes in catalyzing specific reactions. These acids could have potential applications in drug development and green chemistry.
Conclusion
Acids and bases are ubiquitous in chemistry and play a pivotal role in countless applications. By mastering the concepts discussed in this article, AP Chemistry students can develop a strong foundation in this essential area of chemistry and prepare themselves for success in their studies and future careers.