In the realm of chemistry, the interplay between anions and cations governs a vast array of phenomena. Understanding the relationship between their radii is paramount in unraveling the intricate behavior of ionic compounds. This article delves into the fundamental principles governing anion radius, its dependence on atomic attraction force and cation ionic radius, and its pivotal role in shaping the properties of ionic materials.

The Nature of Anions and Cations
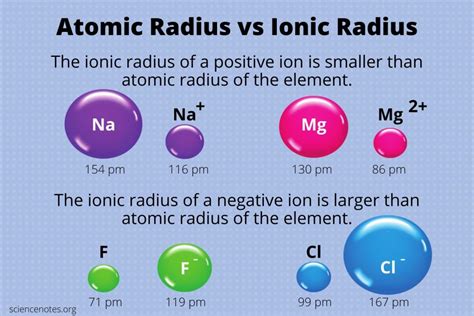
Anions are negatively charged ions, formed when an atom gains one or more electrons. Cations, on the other hand, are positively charged ions, resulting from the loss of electrons. The electrostatic attraction between oppositely charged ions forms the basis for ionic bonding, giving rise to ionic compounds.
Anion Radius: Influenced by Atomic Attraction Force
The anion radius is the distance from the nucleus to the outermost electron shell of an anion. It is primarily influenced by the atomic attraction force, which is the electrostatic force between the positively charged nucleus and the negatively charged electrons.
The atomic attraction force is directly proportional to the number of protons in the nucleus and inversely proportional to the number of electron shells. This means that anions with a higher atomic number (more protons) and fewer electron shells have a stronger atomic attraction force and, consequently, a smaller radius.
Cation Ionic Radius: A Counterbalancing Effect
In the formation of ionic compounds, the size and charge of the cation also play a crucial role in determining the anion radius. The electrostatic attraction between the cation and anion is inversely proportional to the cation ionic radius. Therefore, larger cations with lower charges exert a weaker attraction on anions, allowing them to expand in size.
Applications of Anion Radius
The understanding of anion radius is essential in numerous scientific fields and technological applications, including:
- Crystal Engineering: Anion radius dictates the packing efficiency of ions in crystals, influencing their physical and chemical properties.
- Battery Technology: The size of anions in electrode materials affects the charge storage capacity and lifespan of batteries.
- Drug Design: Anion radius impacts the solubility and bioavailability of pharmaceutical compounds.
- Biomineralization: The formation of bone, teeth, and other biominerals is intricately linked to the radius and charge of anions.
Tables for Anion Radii
| Anion | Radius (pm) |
|---|---|
| Fluoride (F-) | 133 |
| Chloride (Cl-) | 181 |
| Bromide (Br-) | 196 |
| Iodide (I-) | 220 |
| Oxide (O2-) | 140 |
| Sulfide (S2-) | 184 |
| Selenide (Se2-) | 198 |
| Telluride (Te2-) | 221 |
Tips and Tricks
- Consider the Pauli exclusion principle when predicting anion radius. This principle states that no two electrons in an atom can have the same set of quantum numbers, which limits the number of electrons in each electron shell and influences the anion size.
- Use reference tables or databases to obtain accurate anion radius values for specific elements.
- Remember that the anion radius can vary slightly depending on the crystal structure and coordination environment of the anion.
FAQs
Q: Why are anions generally larger than cations?
A: Anions have a net negative charge, which increases the electron-electron repulsion and weakens the atomic attraction force, leading to a larger radius.
Q: How does the anion radius influence the solubility of ionic compounds?
A: Smaller anions tend to form more soluble ionic compounds because they pack more efficiently in water molecules.
Q: What is the relationship between anion radius and lattice energy?
A: Anion radius is inversely proportional to lattice energy. Smaller anions have a stronger electrostatic attraction with cations, resulting in higher lattice energy.
Q: How can anion radius be experimentally determined?
A: Anion radius can be determined using techniques such as X-ray crystallography, neutron diffraction, and ion mobility measurements.