Introduction: The Art of Acid-Base Chemistry
Acid-base titration, a cornerstone of analytical chemistry, empowers scientists to determine the unknown concentration of an acid or base using a known solution of the other. This technique finds widespread application in various fields, including:

- Environmental monitoring: Detecting acid rain or determining water quality
- Industrial chemistry: Controlling chemical reactions or optimizing production processes
- Medical diagnostics: Analyzing blood samples or testing for drug concentrations
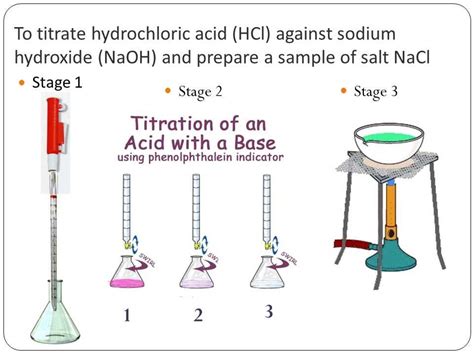
Acid-Base Titration: A Step-by-Step Guide
Materials Required
- Unknown acid or base solution
- Known acid or base solution (standard solution)
- Buret
- Pipet
- Phenolphthalein indicator
- Erlenmeyer flask
Procedure
- Prepare a clean buret: Rinse the buret with distilled water and then with the standard solution. Fill the buret with the standard solution to a specific volume.
- Measure the unknown solution: Use a pipet to accurately measure a known volume of the unknown solution into an Erlenmeyer flask.
- Add indicator: Add a few drops of phenolphthalein indicator to the unknown solution.
- Start titration: Slowly add the standard solution from the buret to the unknown solution while gently swirling the flask.
- Monitor endpoint: Observe the solution until a color change occurs (e.g., from colorless to pink for phenolphthalein). This color change indicates the equivalence point.
- Record the volume of standard solution used: Note the volume of standard solution added from the buret to reach the equivalence point.
Data Analysis: Unraveling the Concentration Mystery
The equivalence point in titration represents the point where the moles of acid are equal to the moles of base added. This relationship allows us to calculate the unknown concentration using the formula:
Concentration of unknown = (Concentration of standard solution × Volume of standard solution) / Volume of unknown
Table 1: Example Data Analysis
| Measurement | Value |
|---|---|
| Concentration of standard solution | 0.1 M |
| Volume of standard solution used | 15.00 mL |
| Volume of unknown solution | 10.00 mL |
Unknown Concentration:
= (0.1 M × 15.00 mL) / 10.00 mL = 0.15 M
Applications Beyond the Lab: Unlocking Uncharted Territories
The principles of acid-base titration extend beyond the confines of the laboratory, offering endless possibilities for innovation:
- Food chemistry: Determining the acidity or alkalinity of food products and beverages
- Pharmaceutical industry: Measuring drug concentrations and ensuring quality control
- Environmental remediation: Evaluating the effectiveness of neutralization processes for acid spills
- Education: Enhancing student understanding of chemical equilibrium and reaction stoichiometry
Conclusion: A Cornerstone of Chemical Knowledge
Acid-base titration stands as a cornerstone of chemical analysis, empowering scientists to unravel the intricacies of acid-base chemistry. With its versatile applications and straightforward principles, this technique continues to serve as an indispensable tool in various fields, paving the way for advancements and discoveries in science and beyond.