In the vast world of chemistry, precision and accuracy govern every experiment, calculation, and discovery. Units, the fundamental building blocks of measurement, play a pivotal role in ensuring the reliability and reproducibility of scientific endeavors. Understanding and using units correctly are paramount for any chemist or student aspiring to unravel the complexities of the chemical realm.

The International System of Units (SI)
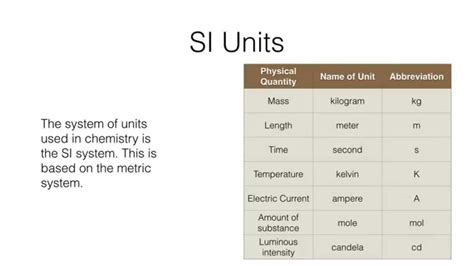
The International System of Units (SI), established by the General Conference on Weights and Measures (CGPM), has become the universally accepted language of measurement in chemistry. SI consists of seven base units:
- Meter (m): Length
- Kilogram (kg): Mass
- Second (s): Time
- Ampere (A): Electric current
- Kelvin (K): Temperature
- Mole (mol): Amount of substance
- Candela (cd): Luminous intensity
These base units are combined to form derived units, which express quantities derived from the base units. For example, velocity is expressed in meters per second (m/s), and volume is expressed in cubic meters (m³).
Fundamental and Derived Units in Chemistry
Chemists primarily utilize the following fundamental and derived SI units:
- Mass: Kilogram (kg)
- Volume: Cubic meter (m³), Liter (L)
- Temperature: Kelvin (K), Celsius (°C)
- Concentration: Molarity (M), Parts per million (ppm)
- Energy: Joule (J)
- Rate: Mole per second (mol/s)
- Equilibrium constant: No units
Metrics and Metric Prefixes
The SI system employs metric prefixes to denote multiples or submultiples of units. These prefixes are:
| Prefix | Symbol | Multiple |
|---|---|---|
| Tera | T | 10¹² |
| Giga | G | 10⁹ |
| Mega | M | 10⁶ |
| Kilo | k | 10³ |
| Centi | c | 10⁻² |
| Milli | m | 10⁻³ |
| Micro | µ | 10⁻⁶ |
| Nano | n | 10⁻⁹ |
| Pico | p | 10⁻¹² |
For example, a megagram (Mg) is equivalent to 10⁶ grams, and a microgram (µg) is equivalent to 10⁻⁶ grams.
Units of Amount of Substance and Chemical Formula Mass
The mole, the SI unit of amount of substance, represents a specific number of elementary entities (atoms, molecules, ions, or electrons). One mole of a substance contains 6.02214076 × 10²³ entities, known as Avogadro’s number.
The chemical formula mass of a compound is the sum of the atomic masses of its constituent elements. It is expressed in grams per mole (g/mol) and is used to convert between the mass and amount of a substance.
Concentrations and Solutions
Chemists often express the concentration of solutions using molarity (M). Molarity is defined as the number of moles of solute per liter of solution:
Molarity = Number of moles of solute / Volume of solution (in liters)
Other units of concentration include:
- Parts per million (ppm): Parts of solute per million parts of solution
- Parts per billion (ppb): Parts of solute per billion parts of solution
Units of Energy and Rate
Energy, the ability to do work, is expressed in joules (J). Changes in energy are often measured in kilojoules (kJ) or megajoules (MJ).
The rate of a chemical reaction is the change in concentration of reactants or products per unit time. It is expressed in moles per second (mol/s).
Units of Equilibrium Constants
Equilibrium constants, which express the relative amounts of reactants and products at equilibrium, do not have units. This is because equilibrium constants are ratios of concentrations, and any units cancel out.
Importance of Units in Chemistry
The correct use of units in chemistry ensures:
- Accuracy: Consistency and reliability in measurements and calculations
- Reproducibility: Ability to replicate experiments and obtain similar results
- Communication: Clear and unambiguous exchange of scientific information
- Standardization: Adherence to universally accepted measurement standards
Tables of Useful Units
Table 1: Base SI Units
| Quantity | Unit | Symbol |
|---|---|---|
| Length | Meter | m |
| Mass | Kilogram | kg |
| Time | Second | s |
| Electric current | Ampere | A |
| Temperature | Kelvin | K |
| Amount of substance | Mole | mol |
| Luminous intensity | Candela | cd |
Table 2: Derived Units in Chemistry
| Quantity | Unit | Symbol |
|---|---|---|
| Volume | Cubic meter | m³ |
| Volume (alternative) | Liter | L |
| Mass (alternative) | Gram | g |
| Concentration | Molarity | M |
| Energy | Joule | J |
| Rate | Mole per second | mol/s |
Table 3: Metric Prefixes
| Prefix | Symbol | Multiple |
|---|---|---|
| Tera | T | 10¹² |
| Giga | G | 10⁹ |
| Mega | M | 10⁶ |
| Kilo | k | 10³ |
| Centi | c | 10⁻² |
| Milli | m | 10⁻³ |
| Micro | µ | 10⁻⁶ |
| Nano | n | 10⁻⁹ |
| Pico | p | 10⁻¹² |
Table 4: Concentrations
| Concentration Unit | Expression |
|---|---|
| Molarity (M) | Number of moles of solute per liter of solution |
| Parts per million (ppm) | Parts of solute per million parts of solution |
| Parts per billion (ppb) | Parts of solute per billion parts of solution |
Tips and Tricks for Working with Units
- Always include units in your measurements and calculations.
- Convert between units as needed to ensure consistency.
- Use dimensional analysis as a tool to check the validity of your calculations.
- Avoid unnecessary precision in your measurements.
- Be aware of the limitations of your measuring instruments.
- Use the correct number of significant figures in your calculations.
Applications of Units in Chemistry
Units are not just abstract concepts but have tangible applications in various fields of chemistry, including:
- Analytical chemistry: Determining the concentration of substances in samples
- Physical chemistry: Studying the properties of matter, such as density and solubility
- Inorganic chemistry: Characterizing chemical compounds and their reactivity
- Organic chemistry: Synthesizing and analyzing organic molecules
- Biochemistry: Investigating the chemical processes in living organisms
Conclusion
Units in chemistry serve as the foundation for precise measurements, accurate calculations, and effective communication. The International System of Units (SI) provides a standardized system of units that enables scientists to conduct research and share their findings with confidence. Understanding the principles and applications of units empowers chemists to advance scientific knowledge and contribute to technological advancements that benefit society.