Introduction
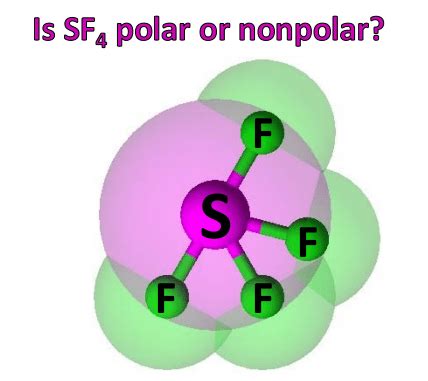
Sulfur tetrafluoride (SF4) is an inorganic compound with the molecular formula SF4. It is a colorless, non-flammable, and toxic gas that is used in a variety of industrial and medical applications. SF4 is a polar molecule, meaning that it has a permanent dipole moment. This polarity is due to the electronegativity difference between sulfur and fluorine.
Structure and Bonding
SF4 has a tetrahedral molecular geometry. The sulfur atom is located at the center of the tetrahedron, and the four fluorine atoms are located at the corners. The sulfur-fluorine bond length is 1.56 Å. The bond angle between the sulfur and fluorine atoms is 109.5°.
The bonding in SF4 can be described using valence bond theory. In this theory, the sulfur atom uses its four valence electrons to form four single bonds with the four fluorine atoms. The fluorine atoms each contribute one valence electron to the bond.
Physical Properties
SF4 is a colorless, non-flammable, and toxic gas. It has a boiling point of -63.8 °C and a melting point of -120.6 °C. SF4 is denser than air, with a density of 6.14 g/L. It is soluble in water, with a solubility of 1.2 g/L at 25 °C.
Chemical Properties
SF4 is a reactive compound. It reacts with water to form hydrogen fluoride and sulfur dioxide. It also reacts with bases to form metal fluorides and hydrogen sulfide.
SF4 is a strong oxidizing agent. It can oxidize many metals, including iron, copper, and aluminum. It can also oxidize organic compounds, such as hydrocarbons and alcohols.
Applications
SF4 is used in a variety of industrial and medical applications. It is used as a dielectric gas in high-voltage electrical equipment. It is also used as a refrigerant in refrigeration systems.
In medicine, SF4 is used as a contrast agent in X-ray imaging. It is also used as an anesthetic in veterinary medicine.
Toxicity
SF4 is a toxic gas. It can cause irritation to the eyes, nose, and throat. It can also cause respiratory problems, such as coughing and shortness of breath. In high concentrations, SF4 can be fatal.
The Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit (PEL) for SF4 of 1 ppm (parts per million) in air. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) for SF4 of 0.1 ppm in air.
Conclusion
SF4 is a polar molecule with a tetrahedral molecular geometry. It is a colorless, non-flammable, and toxic gas that is used in a variety of industrial and medical applications. SF4 is a reactive compound that can react with water, bases, and metals. It is also a strong oxidizing agent. SF4 is a toxic gas that can cause irritation to the eyes, nose, and throat. It can also cause respiratory problems. OSHA has set a PEL for SF4 of 1 ppm in air, and NIOSH has set a REL for SF4 of 0.1 ppm in air.
- Dielectric gas in high-voltage electrical equipment
- Refrigerant in refrigeration systems
- Contrast agent in X-ray imaging
- Anesthetic in veterinary medicine
- Non-flammable
- High dielectric strength
- Low thermal conductivity
- High chemical stability
- Toxic
- Expensive
- Greenhouse gas
Several alternatives to SF4 are available, including:
- Hydrofluorocarbons (HFCs)
- Perfluorocarbons (PFCs)
- Sulfur hexafluoride (SF6)
- Nitrogen (N2)
These alternatives have different properties than SF4, so it is important to carefully consider the application before selecting an alternative.
Table 1: Physical Properties of SF4
| Property | Value |
|---|---|
| Molecular weight | 108.09 g/mol |
| Boiling point | -63.8 °C |
| Melting point | -120.6 °C |
| Density | 6.14 g/L |
| Solubility in water | 1.2 g/L at 25 °C |
Table 2: Chemical Properties of SF4
| Property | Value |
|---|---|
| Reactivity | Reactive |
| Reactions | Reacts with water, bases, and metals |
| Oxidizing agent | Strong |
Table 3: Applications of SF4
| Application | Description |
|---|---|
| Dielectric gas | Used in high-voltage electrical equipment |
| Refrigerant | Used in refrigeration systems |
| Contrast agent | Used in X-ray imaging |
| Anesthetic | Used in veterinary medicine |
Table 4: Alternatives to SF4
| Alternative | Properties |
|---|---|
| Hydrofluorocarbons (HFCs) | Non-flammable, low dielectric strength, high thermal conductivity, low chemical stability |
| Perfluorocarbons (PFCs) | Non-flammable, high dielectric strength, low thermal conductivity, high chemical stability |
| Sulfur hexafluoride (SF6) | Non-flammable, high dielectric strength, low thermal conductivity, high chemical stability |
| Nitrogen (N2) | Non-flammable, low dielectric strength, low thermal conductivity, high chemical stability |