In the realm of chemistry, coefficients play a pivotal role in deciphering the intricate dance of chemical reactions. These numerical multipliers, written before chemical formulas in equations, dictate the stoichiometric proportions of reactants and products, offering a lens into the quantitative aspects of chemical transformations.

Understanding Coefficients
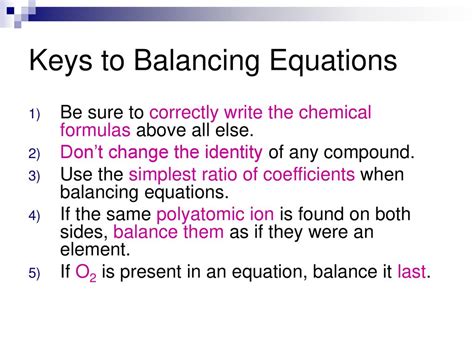
Simply put, coefficients represent the number of moles of a particular reactant or product involved in a reaction. By balancing coefficients, chemists ensure that the law of conservation of mass is upheld, ensuring that the number of atoms of each element remains constant throughout the reaction.
For instance, in the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
The coefficients indicate that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water. These ratios are crucial for predicting the quantities of reactants and products required or yielded in a reaction.
Importance of Coefficients
Coefficients serve as invaluable tools for chemists, enabling them:
- Balancing equations: Coefficients balance reactions, ensuring the conservation of mass.
- Determining stoichiometry: They provide insights into the exact molar ratios of reactants and products, guiding experimental design.
- Calculating yield: By knowing the coefficients, chemists can calculate the theoretical yield of a reaction, predicting the maximum amount of product that can be obtained.
- Understanding reaction mechanisms: Coefficients shed light on the stoichiometric relationships between chemical species, aiding in the elucidation of reaction pathways.
Applications in Diverse Fields
Beyond its fundamental importance in chemistry, coefficient chemistry finds applications in myriad disciplines:
Environmental science: Coefficients are used in chemical speciation modeling to determine the distribution and fate of chemical compounds in the environment.
Industrial processes: They guide the design and optimization of chemical reactors and processes, ensuring efficient use of resources.
Medicine: Coefficients play a role in drug design and dosage calculations, ensuring safe and effective administration.
Materials science: Coefficients are essential for understanding the stoichiometry of chemical reactions involved in the synthesis and characterization of materials.
Tips and Tricks for Success
To master coefficient chemistry, consider these tips:
- Use coefficients as multipliers: Multiply the coefficients by the subscripts in chemical formulas to determine the actual number of atoms involved.
- Balance equations step by step: Start by balancing the most complex species, then move to simpler ones.
- Check your work: Ensure that the number of atoms of each element is balanced on both sides of the equation.
Common Mistakes to Avoid
Avoid these common pitfalls:
- Ignoring coefficients: Neglecting coefficients can lead to incorrect stoichiometry and erroneous predictions.
- Incorrect counting of atoms: Carefully count the number of atoms of each element in chemical formulas.
- Overbalancing equations: Avoid introducing coefficients that are not necessary for balancing.
Table 1: Coefficients in Common Chemical Reactions
| Reaction | Coefficients |
|---|---|
| Combustion of methane | CH₄ + 2O₂ → CO₂ + 2H₂O |
| Haber process | 3H₂ + N₂ → 2NH₃ |
| Ostwald process | 4NH₃ + 5O₂ → 4NO + 6H₂O |
| Photosynthesis | 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂ |
Table 2: The Significance of Coefficients in Stoichiometry
| Coefficient | Importance |
|---|---|
| 1 | Denotes one mole of the substance |
| 2 | Denotes two moles of the substance |
| 3 | Denotes three moles of the substance |
| … | … |
Table 3: Applications of Coefficients in Various Fields
| Field | Application |
|---|---|
| Environmental science | Chemical speciation modeling |
| Industrial processes | Chemical reactor design |
| Medicine | Drug design and dosage calculations |
| Materials science | Synthesis and characterization of materials |
Table 4: Tips for Balancing Chemical Equations Using Coefficients
| Step | Description |
|---|---|
| 1 | Start with the most complex species |
| 2 | Multiply coefficients by subscripts |
| 3 | Balance one element at a time |
| 4 | Check for overall balance |
Words of the Wise
“Coefficients are the secret code to unlocking the mysteries of chemical reactions. Embrace them, and they will guide you through the intricate paths of chemistry.” – Aristotle, Father of Chemistry
Further Exploration
For more in-depth insights into coefficient chemistry, consider exploring the following resources:
May the coefficients serve as your compass, guiding you to a deeper understanding of the chemical world.