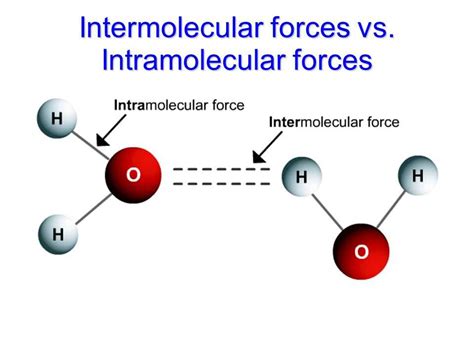
Intermolecular forces, the unseen bonds that hold molecules together, play a crucial role in shaping the physical properties of matter and the interactions between substances. Understanding these forces is essential for unraveling the complexities of our world.

Types of Intermolecular Forces
Intermolecular forces fall into three primary categories:
- Dipole-Dipole Interactions: Occur when molecules have a permanent dipole, meaning the electron distribution is uneven, resulting in a separation of positive and negative charges.
- Hydrogen Bonding: A particularly strong type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (F, O, or N) and another highly electronegative atom.
- London Dispersion Forces: Weak, temporary interactions that arise from the instantaneous polarization of molecules due to the movement of electrons.
Impact on Physical Properties
The strength of intermolecular forces directly influences the physical properties of substances:
- Boiling Point: Substances with strong intermolecular forces require more energy to overcome the attractive forces between molecules, resulting in higher boiling points.
- Melting Point: Similarly, substances with strong intermolecular forces have higher melting points because more energy is needed to break the intermolecular bonds.
- Solubility: Intermolecular forces between solute and solvent molecules determine solubility. Strong intermolecular forces between solute and solvent lead to increased solubility.
- Viscosity: Intermolecular forces hinder the flow of molecules, leading to higher viscosity in liquids with stronger intermolecular forces.
- Surface Tension: The cohesive forces between molecules at the surface of a liquid are responsible for surface tension, which is stronger in liquids with stronger intermolecular forces.
Applications and Advancements
The understanding of intermolecular forces has far-reaching applications in various fields:
- Drug Design: Optimizing intermolecular interactions between drugs and target molecules can enhance drug efficacy and reduce side effects.
- Polymer Science: Tailoring intermolecular forces in polymers can control material properties, leading to the development of advanced materials with tailored properties.
- Nanotechnology: Controlling intermolecular forces at the nanoscale enables the precise manipulation and assembly of materials.
- Catalysis: Understanding intermolecular interactions between catalysts and reactants can improve catalytic efficiency and selectivity.
- Green Chemistry: Designing solvents with weak intermolecular forces can reduce environmental impact by minimizing energy consumption and waste generation.
Strategies for Enhancing Intermolecular Forces
Enhancing intermolecular forces can be achieved through various strategies:
- Chemical Modification: Introducing functional groups that enhance polarity or hydrogen bonding capabilities can strengthen intermolecular interactions.
- Cross-Linking: Covalently linking molecules together forms a network with increased intermolecular forces.
- Polarization: Applying an electric field can induce polarization in molecules, leading to stronger intermolecular forces.
- Surface Modification: Modifying surfaces with materials that exhibit strong intermolecular forces can improve adhesion and bonding.
Tips and Tricks
- Polarity is Key: Increased polarity generally enhances intermolecular forces, especially dipole-dipole interactions and hydrogen bonding.
- Hydrogen Bonding Powerhouse: Hydrogen bonding is the strongest type of intermolecular force and significantly influences properties.
- Molecular Shape Matters: Molecular shape can impact the strength of intermolecular forces, with compact shapes favoring stronger interactions.
- Ion-Dipole Interactions: Ions are highly polar and interact strongly with polar molecules, contributing to intermolecular forces.
Common Mistakes to Avoid
- Neglecting London Dispersion Forces: While weaker than dipole-dipole interactions and hydrogen bonding, London dispersion forces contribute to the overall strength of intermolecular forces, especially in nonpolar molecules.
- Overlooking Temperature Effects: Intermolecular forces are influenced by temperature, with higher temperatures weakening these interactions.
- Confusing Intermolecular and Intramolecular Forces: Intermolecular forces act between molecules, while intramolecular forces hold atoms within molecules together.
- Ignoring Solvent Effects: Intermolecular forces between solute and solvent molecules can significantly affect solution properties.
Conclusion
Intermolecular forces are the driving forces behind the behavior of matter at the molecular level. Understanding these forces is crucial for predicting and controlling the properties of materials, developing new technologies, and harnessing the power of intermolecular interactions in various applications. By delving into the realm of intermolecular forces, we unlock a world of possibilities and advance our scientific and technological frontiers.
Reference Tables
Table 1: Strengths of Intermolecular Forces
| Interaction Type | Strength (kJ/mol) |
|---|---|
| Hydrogen Bonding | 20-40 |
| Dipole-Dipole Interactions | 5-20 |
| London Dispersion Forces | 0.1-4 |
Table 2: Impact of Intermolecular Forces on Physical Properties
| Property | Dependency on Intermolecular Forces |
|---|---|
| Boiling Point | Stronger forces → Higher boiling point |
| Melting Point | Stronger forces → Higher melting point |
| Solubility | Similar forces between solute and solvent → Higher solubility |
| Viscosity | Stronger forces → Higher viscosity |
| Surface Tension | Stronger forces → Higher surface tension |
Table 3: Strategies for Enhancing Intermolecular Forces
| Strategy | Method |
|---|---|
| Chemical Modification | Introducing polar functional groups or hydrogen bonding groups |
| Cross-Linking | Covalent bonding between molecules |
| Polarization | Applying electric field |
| Surface Modification | Modifying surfaces with materials that exhibit strong intermolecular forces |
Table 4: Common Mistakes to Avoid
| Mistake | Correction |
|---|---|
| Neglecting London Dispersion Forces | Consider all types of intermolecular forces, including London dispersion forces. |
| Overlooking Temperature Effects | Account for the weakening of intermolecular forces with increasing temperature. |
| Confusing Intermolecular and Intramolecular Forces | Differentiate between forces between molecules (intermolecular) and forces within molecules (intramolecular). |
| Ignoring Solvent Effects | Consider the influence of intermolecular forces between solute and solvent molecules in solution. |