Introduction: Understanding Electrolytes and Their Unique Properties
In the realm of chemistry, certain substances possess the remarkable ability to conduct electricity when dissolved in water. Known as electrolytes, these compounds play a vital role in numerous biological processes and industrial applications. This article delves into the fascinating world of electrolytes, exploring their properties, applications, and the profound implications they have in various scientific disciplines.

Definition and Key Characteristics of Electrolytes
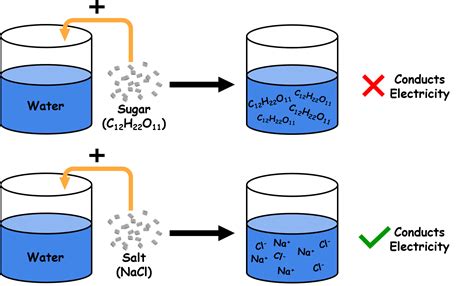
An electrolyte is a substance that, when dissolved in water, dissociates into ions, positively charged cations, and negatively charged anions. This dissociation process enables the solution to conduct electricity. The conductivity of an electrolyte solution depends on the number of ions present and their mobility. Strong electrolytes, such as sodium chloride (NaCl), dissociate completely in water, resulting in high conductivity. Weak electrolytes, on the other hand, dissociate only partially, leading to lower conductivity.
Electrolyte Solutions in Biological Systems
Electrolyte solutions are essential for maintaining homeostasis and proper physiological function. They regulate electrical signals in the body, facilitate nutrient transport, and control fluid balance. For instance, potassium ions (K+) play a crucial role in nerve conduction, while sodium ions (Na+) and chloride ions (Cl-) contribute to fluid balance and muscle contraction.
Industrial Applications of Electrolytes
In the industrial sector, electrolytes find widespread use in various applications, including:
- Batteries: Electrolytes provide a medium for the movement of ions between electrodes, enabling the storage and release of electrical energy.
- Electroplating: Electrolyte solutions are used to deposit metal ions onto surfaces for various purposes, such as corrosion protection and decorative finishes.
- Electrochemical sensors: Electrolyte solutions are employed in sensors to detect and analyze specific ions in various settings, such as environmental monitoring and medical diagnostics.
Novel Applications of Electrolytes
Recent advancements in research have led to the discovery of novel properties and applications of electrolytes:
- Electrolyte-gated transistors: Transistors based on electrolyte solutions show promise for high-performance computing and neuromorphic devices.
- Flexible batteries: Electrolyte solutions can be incorporated into flexible devices for wearable electronics and portable power sources.
- Self-healing materials: Electrolytes can be used to create self-healing materials by enabling the mobility of ions to repair damage.
Benefits of Using Electrolyte Solutions
The use of electrolyte solutions offers numerous benefits in various applications:
- High conductivity: Electrolyte solutions exhibit high electrical conductivity, enabling efficient ion transport and energy transfer.
- Versatility: Electrolytes can be tailored to meet specific requirements, such as temperature stability, pH stability, and compatibility with different electrodes.
- Scalability: Electrolyte solutions can be easily produced and scaled up for large-scale applications, such as grid-scale energy storage.
Challenges and Considerations
Despite their numerous benefits, electrolyte solutions also pose certain challenges:
- Corrosion: Electrolytes can be corrosive to electrodes and other components, requiring careful material selection and corrosion protection measures.
- Toxicity: Some electrolytes, such as strong acids and bases, can be toxic and require proper handling and disposal.
- Thermal stability: Electrolyte solutions may degrade at elevated temperatures, limiting their use in high-temperature applications.
Effective Strategies for Electrolyte Optimization
To maximize the performance and reliability of electrolyte solutions, several effective strategies can be employed:
- Selection of suitable electrolytes: Choosing electrolytes that are stable, compatible with electrodes, and meet the specific application requirements is crucial.
- Additives and modifiers: Additives can be used to improve electrolyte properties, such as conductivity, corrosion resistance, and thermal stability.
- Electrode surface modification: Modifications to electrode surfaces can enhance ion transfer and reduce corrosion.
Step-by-Step Approach to Electrolyte Solution Preparation
- Calculate the required concentration: Determine the desired concentration of the electrolyte solution based on the application requirements.
- Dissolve the electrolyte: Gradually add the electrolyte to water while stirring to ensure complete dissolution.
- Filter the solution: Filter the solution to remove any impurities or undissolved particles.
- Test the conductivity: Measure the conductivity of the solution using a conductivity meter to ensure it meets the desired value.
Why Electrolytes Matter: Implications in Science and Engineering
Electrolytes play a fundamental role in understanding and controlling electrical processes in various scientific and engineering fields:
- Electrochemistry: Electrolytes are the basis of electrochemical cells, enabling the study of redox reactions and energy conversion.
- Materials science: Electrolytes are used to synthesize and characterize new materials, such as nanomaterials and semiconductors.
- Environmental science: Electrolytes are essential for monitoring and remediating environmental pollutants.
Conclusion: The Future of Electrolytes
Electrolytes are indispensable materials with wide-ranging applications across multiple disciplines. As research continues to unveil their potential, novel applications and advancements are expected to emerge, further expanding their role in shaping the future of science and engineering.
Additional Resources
- International Union of Pure and Applied Chemistry (IUPAC)
- National Institute of Standards and Technology (NIST)
- Electrolytes in Batteries
Tables
Table 1: Common Electrolytes and Their Properties
| Electrolyte | Formula | Dissociation | Conductivity (mS/cm) |
|---|---|---|---|
| Sodium chloride | NaCl | Complete | 59.6 |
| Potassium chloride | KCl | Complete | 39.1 |
| Sulfuric acid | H2SO4 | Complete | 18.0 |
| Acetic acid | CH3COOH | Partial | 1.8 |
Table 2: Applications of Electrolytes in Biological Systems
| Process | Electrolyte | Function |
|---|---|---|
| Nerve conduction | Potassium ions (K+) | Transmission of electrical signals |
| Muscle contraction | Sodium ions (Na+) and chloride ions (Cl-) | Regulation of muscle movement |
| Nutrient transport | Various ions | Facilitating the movement of nutrients into and out of cells |
Table 3: Industrial Applications of Electrolytes
| Application | Electrolyte | Role |
|---|---|---|
| Batteries | Lithium-ion electrolytes | Energy storage and release |
| Electroplating | Metal ion solutions | Deposition of metal ions onto surfaces |
| Sensors | Ion-selective electrolytes | Detection and analysis of specific ions |
Table 4: Challenges and Considerations for Electrolyte Solutions
| Challenge | Consideration |
|---|---|
| Corrosion | Careful material selection and corrosion protection |
| Toxicity | Proper handling and disposal |
| Thermal stability | Selection of electrolytes with suitable temperature stability |