Water holds the distinction of being the most abundant compound on Earth. Its significance extends far beyond its prevalence, as it plays a pivotal role in the sustenance of life. A deeper understanding of water’s molecular structure can pave the way for groundbreaking advancements in various fields, spanning chemistry, biology, and environmental sciences.

Delving into the Concept of Lewis Dot Diagrams
To unravel the intricacies of water’s molecular structure, we must first delve into the concept of Lewis dot diagrams. This visual representation portrays the arrangement of electrons within a molecule. Each atom is represented by its chemical symbol, and the valence electrons (those involved in chemical bonding) are denoted by dots.
Key Points of Lewis Dot Diagrams:
- Valence electrons are depicted as dots surrounding the chemical symbol.
- Atoms strive to achieve a stable electron configuration, typically by gaining or losing electrons.
- The number of valence electrons determines an atom’s chemical reactivity.
Lewis Dot Diagram for Water
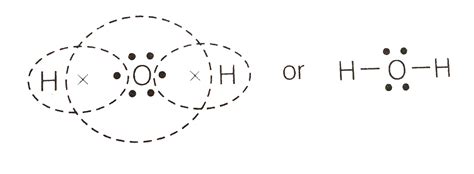
Water’s Lewis dot diagram provides a simplified yet powerful representation of its molecular structure. Following the principles outlined above, we can construct the Lewis dot diagram for water as follows:
H
|
H-O-H
|
H
This diagram reveals several essential features of the water molecule:
Lone Pairs and Bonding Pairs
- Each hydrogen atom has one valence electron, which it contributes to a covalent bond with the oxygen atom.
- Oxygen has six valence electrons, two of which form covalent bonds with hydrogen atoms.
- The remaining four valence electrons reside as two lone pairs on the oxygen atom.
Molecular Geometry
- The Lewis dot diagram suggests a tetrahedral electron-pair geometry around the oxygen atom.
- The two lone pairs and two bonding pairs repel each other, resulting in a bent molecular geometry.
- The H-O-H bond angle is approximately 104.5 degrees.
Significance of Water’s Molecular Structure
The specific arrangement of atoms and electrons in water gives rise to its unique properties, which are essential for life on Earth:
- Polarity: Water molecules possess a dipole moment due to the uneven distribution of electrons. This polarity enables water to interact with polar molecules and ions.
- Hydrogen Bonding: The lone pairs on the oxygen atom can form hydrogen bonds with other water molecules or other polar molecules. Hydrogen bonding is responsible for water’s high boiling point and surface tension.
- High Specific Heat Capacity: The hydrogen bonding in water allows it to absorb and release large amounts of heat without experiencing significant temperature changes. This property contributes to the Earth’s climate regulation.
Applications of Water’s Molecular Structure
A thorough understanding of water’s molecular structure has far-reaching implications and has led to numerous practical applications:
- Water Purification: Understanding water’s polarity and hydrogen bonding mechanisms facilitates the development of efficient water purification technologies.
- Drug Design: The polarity and hydrogen bonding capabilities of water play a crucial role in drug design, as they influence the solubility and bioavailability of pharmaceutical compounds.
- Materials Science: The unique properties of water, such as its surface tension and capillary action, have inspired the creation of innovative materials with tailored properties.
Advanced Applications for the Future
The exploration of water’s molecular structure continues to yield novel insights and potential applications:
- Quantum Computing: Hydrogen bonding in water molecules may serve as a potential building block for quantum computing systems.
- Water-Based Energy Storage: The hydrogen bonding in water can be exploited to design water-based energy storage systems.
- Water-Responsive Biomaterials: The development of water-responsive biomaterials that can interact with water in specific ways holds promise for advancing biomedical technologies.
Impact of Water’s Molecular Structure on Various Industries
The knowledge gained from water’s molecular structure has a profound impact on a diverse range of industries, including:
- Agriculture: Understanding water’s properties is essential for optimizing irrigation practices and improving crop yield.
- Pharmaceuticals: The structure of water guides the design and delivery of drugs.
- Environmental Science: Water’s molecular structure plays a vital role in understanding and addressing water pollution and climate change.
FAQs
-
What is a Lewis dot diagram?
– A Lewis dot diagram is a visual representation of the arrangement of electrons within a molecule. -
How do I draw a Lewis dot diagram for water?
– The Lewis dot diagram for water shows one oxygen atom bonded to two hydrogen atoms. The oxygen atom has two lone pairs of electrons. -
What is the molecular geometry of water?
– The molecular geometry of water is bent, with a bond angle of approximately 104.5 degrees. -
Why is water polar?
– Water is polar due to the uneven distribution of electrons, which results in a dipole moment. -
What is the significance of water’s molecular structure?
– Water’s molecular structure gives rise to its unique properties, such as polarity, hydrogen bonding, and high specific heat capacity. -
How is the knowledge of water’s molecular structure used in practice?
– The understanding of water’s molecular structure has led to numerous applications, including water purification, drug design, and materials science. -
What are some potential future applications of water’s molecular structure?
– Potential future applications include quantum computing, water-based energy storage, and water-responsive biomaterials. -
How does water’s molecular structure impact various industries?
– Water’s molecular structure plays a crucial role in agriculture, pharmaceuticals, and environmental science, among other industries.