Enzymes, the biological catalysts, play a crucial role in all living organisms. Their ability to transform substances into products is fundamental to life processes. To harness the full potential of enzymes, it is essential to understand the conditions under which they function optimally.

Optimum Temperature
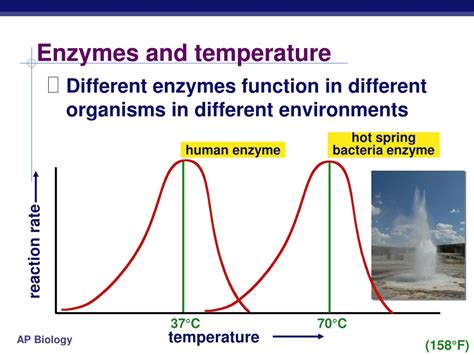
Every enzyme has an optimum temperature at which it exhibits maximum activity. Most enzymes operate within a narrow temperature range, typically between 30°C and 40°C. Deviation from this range can lead to a rapid decline in enzyme activity.
Why Temperature Matters
- Temperature influences the molecular motion of the enzyme and its substrate.
- At optimum temperatures, the enzyme and substrate collide with the correct orientation and energy to facilitate the chemical reaction.
- Extreme temperatures can disrupt enzyme structure and denature the protein, leading to loss of activity.
Optimum pH
Similar to temperature, each enzyme has an optimum pH at which it functions efficiently. Most enzymes operate within a pH range of 6.5 to 8.0. Acidic or alkaline pH levels can compromise enzyme activity.
How pH Benefits
- pH affects the ionization of the enzyme’s active site and the substrate.
- Ionization alters the charge interactions that stabilize the enzyme-substrate complex.
- Optimal pH promotes the correct electrostatic environment for the reaction to occur.
Substrate Concentration
The rate of enzyme-catalyzed reactions is dependent on the concentration of the substrate. Initially, the reaction rate increases linearly with increasing substrate concentration. However, a plateau is reached once the enzyme is saturated with substrate molecules.
Common Mistake to Avoid
Overcrowding the enzyme with substrate can hinder its activity. Avoid saturating the enzyme with excessive substrate to ensure optimal performance.
Cofactors and Coenzymes
Many enzymes require cofactors or coenzymes to function. Cofactors are metal ions or small organic molecules that bind to the enzyme and facilitate its catalytic activity. Coenzymes are organic molecules that undergo chemical changes during the reaction.
Importance of Cofactors and Coenzymes
- Cofactors and coenzymes provide essential functional groups for the enzyme-catalyzed reaction.
- They participate in the transfer of electrons, protons, or functional groups, enabling the enzyme to catalyze specific reactions.
Inhibitors
Inhibitors are molecules that block enzyme function. They can bind to the enzyme’s active site, preventing substrate access, or interfere with enzyme activity in other ways.
Types of Inhibitors
- Competitive inhibitors compete with the substrate for binding to the active site.
- Noncompetitive inhibitors bind to the enzyme at a different site, causing conformational changes that reduce activity.
Step-by-Step Approach to Optimizing Enzyme Function
- Determine the optimum temperature and pH for the enzyme.
- Provide sufficient substrate concentration without overwhelming the enzyme.
- Ensure the presence of necessary cofactors or coenzymes.
- Eliminate potential inhibitors from the reaction system.
Applications of Enzyme Function Optimization
Optimizing enzyme function has far-reaching applications in various industries:
Biotechnology:
* Enhanced efficiency of enzyme-based industrial processes (e.g., biofuel production, wastewater treatment)
* Development of new therapeutic enzymes for treating diseases
Food industry:
* Improved quality and shelf life of food products
* Optimization of enzymatic processes in food processing (e.g., baking, brewing)
Medical diagnostics:
* Faster and more accurate enzyme-based tests for disease detection and monitoring
Bioenergy:
* Enhanced efficiency of enzymes involved in the production of renewable energy sources (e.g., biofuels)
Environmental biotechnology:
* Bioremediation of contaminated sites using enzymes that degrade pollutants
* Optimization of wastewater treatment processes using enzyme-based technologies
Tables for Reference
Table 1: Optimum Temperature Ranges for Common Enzymes
| Enzyme | Optimum Temperature (°C) |
|---|---|
| Amylase | 37-42 |
| Protease | 40-45 |
| Lipase | 35-40 |
| Lactase | 32-37 |
| Glucose oxidase | 25-30 |
Table 2: Optimum pH Ranges for Common Enzymes
| Enzyme | Optimum pH |
|---|---|
| Amylase | 6.0-7.0 |
| Protease | 7.5-8.5 |
| Lipase | 7.0-8.0 |
| Lactase | 6.2-6.8 |
| Glucose oxidase | 5.0-6.0 |
Table 3: Common Cofactors and Coenzymes for Enzymes
| Cofactor/Coenzyme | Description |
|---|---|
| NAD+ | Nicotinamide adenine dinucleotide |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| FAD | Flavin adenine dinucleotide |
| CoA | Coenzyme A |
| Heme | Iron-containing porphyrin ring |
Table 4: Types of Inhibitors and Their Mechanisms
| Inhibitor | Mechanism |
|---|---|
| Competitive | Binds to the active site, competing with the substrate |
| Noncompetitive | Binds to the enzyme at a different site, causing conformational changes |
| Irreversible | Forms a covalent bond with the enzyme, permanently inactivating it |
| Reversible | Forms a noncovalent bond with the enzyme, inhibiting activity temporarily |