Introduction
Distilled water, also known as pure water, is a ubiquitous substance with a wide range of applications in various industries, including medicine, manufacturing, and scientific research. Its unique properties, such as high purity and minimal impurities, make it an essential component in many processes. Understanding the chemical makeup of distilled water is crucial for harnessing its full potential.

Chemical Composition
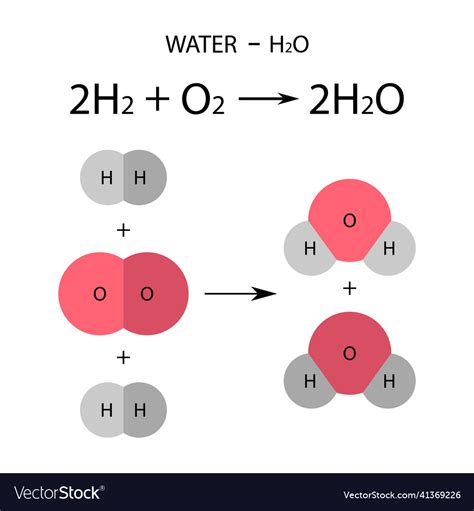
The chemical equation for distilled water is simply:
H2O
This equation represents the combination of two hydrogen atoms (H) with one oxygen atom (O). The hydrogen atoms form covalent bonds with the oxygen atom, creating a stable molecule. Distilled water has a molecular weight of 18.015 g/mol and a density of 1 g/mL at room temperature (25°C).
Properties of Distilled Water
The unique properties of distilled water arise from its chemical composition:
- High Purity: Distilled water contains minimal impurities, including dissolved salts, minerals, and organic matter.
- Neutral pH: Distilled water has a pH of 7, indicating its neutrality.
- Low Electrical Conductivity: Due to its lack of impurities, distilled water has a low electrical conductivity, making it an excellent insulator.
- High Boiling Point: The boiling point of distilled water is 100°C (212°F) at sea level, slightly higher than that of tap water.
- Low Freezing Point: The freezing point of distilled water is 0°C (32°F), similar to tap water.
Applications of Distilled Water
The broad spectrum of applications for distilled water reflects its unique properties:
- Medical: Distilled water is used in intravenous fluids, wound cleaning, and pharmaceutical manufacturing.
- Industrial: It serves as a solvent in various industries, including chemical manufacturing, electronics, and food processing.
- Scientific Research: Distilled water is essential for laboratory experiments, chemical analysis, and calibration procedures.
- Automotive: It is used in car batteries, cooling systems, and windshield washer fluid.
- Household: Distilled water is employed in humidifiers, steam irons, and cleaning solutions.
Advantages of Distilled Water
Compared to tap water, distilled water offers several significant advantages:
- Enhanced Purity: Distilled water is significantly purer, making it ideal for applications where high purity is crucial.
- Extended Shelf Life: The absence of impurities in distilled water prevents microbial growth, extending its shelf life.
- Reduced Corrosion: Distilled water minimizes corrosion in pipes and equipment, as it lacks corrosive salts and minerals.
- Improved Taste: The lack of impurities imparts a neutral taste to distilled water, making it palatable for consumption.
Disadvantages of Distilled Water
While distilled water has numerous benefits, it also comes with certain drawbacks:
- Mineral Deficiency: Distillation removes essential minerals such as calcium and magnesium, making distilled water unsuitable for long-term consumption.
- Higher Cost: Distilled water requires a specific purification process, making it more expensive than tap water.
- Potential Contamination: Distilled water can become contaminated if improperly stored, handling, or packaged.
Conclusion
Distilled water, represented by the chemical equation H2O, is a vital substance with wide-ranging applications. Its unique properties, such as high purity, neutrality, and low electrical conductivity, make it essential in various industries. However, its mineral deficiency and potential for contamination should be considered when using distilled water for certain purposes.