Introduction
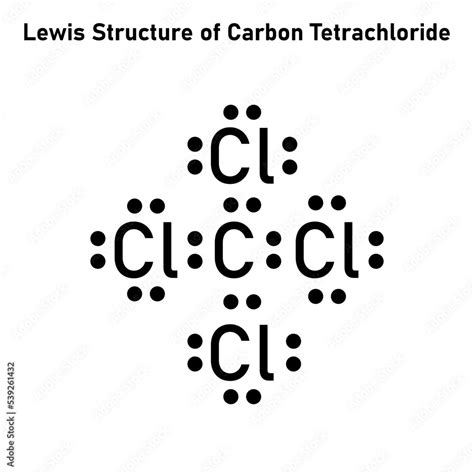
Carbon tetrachloride (CCl4) is an organic compound known for its nonpolar and highly symmetrical structure. Its Lewis structure, which represents the distribution of electrons within the molecule, plays a crucial role in understanding its chemical properties and applications. This guide delves into the intricacies of the carbon tetrachloride Lewis structure, providing insights into its bonding, shape, and reactivity.

Bonding and Structure
The Lewis structure of carbon tetrachloride depicts a central carbon atom surrounded by four chlorine atoms, arranged tetrahedrally around it. Each carbon-chlorine bond is formed by the sharing of two electrons, resulting in a total of four covalent bonds in the molecule.
Properties of Carbon Tetrachloride
Physical Properties:
- Colorless liquid at room temperature
- Boiling point: 76.5 °C (170 °F)
- Melting point: -22.8 °C (-9 °F)
- Density: 1.59 g/cm³ at 20 °C
Chemical Properties:
- Nonpolar: The tetrahedral arrangement of chlorine atoms around the carbon atom creates a symmetrical distribution of electrons, resulting in a zero dipole moment.
- Inert: The strong carbon-chlorine bonds make the molecule highly resistant to chemical reactions, giving it the nickname “carbon tet.”
Applications
Carbon tetrachloride has been widely used in various industries, but its applications have declined due to its environmental concerns. It was once commonly employed as:
- A cleaning agent for dry cleaning and degreasing metals
- A solvent for dyes and oils
- A fire extinguisher in fire extinguishers
Environmental Impact and Safety Concerns
Carbon tetrachloride is a toxic substance that poses significant environmental and health risks.
- Toxicity: Inhalation or ingestion of carbon tetrachloride can lead to severe liver and kidney damage. It is also a possible carcinogen.
- Ozone Depletion: Carbon tetrachloride contributes to ozone depletion in the stratosphere.
- Groundwater Contamination: Improper disposal of carbon tetrachloride can contaminate groundwater sources.
Tips and Tricks
- When drawing the Lewis structure of carbon tetrachloride, remember that the central carbon atom should have four single bonds to the chlorine atoms.
- The tetrahedral arrangement of chlorine atoms results in bond angles of approximately 109.5 degrees.
- Carbon tetrachloride is highly flammable, so it should be handled with caution and only in well-ventilated areas.
Common Mistakes to Avoid
- Do not overcrowd the central carbon atom with more than four bonds.
- Do not underestimate the polarity of the molecule. Although carbon tetrachloride is nonpolar overall, there is a slight dipole moment between the carbon and chlorine atoms.
- Do not handle carbon tetrachloride without proper safety precautions, as it is a toxic substance.
FAQs
-
What is the molecular weight of carbon tetrachloride?
153.82 g/mol -
What is the solubility of carbon tetrachloride in water?
Almost insoluble (0.01 g/100 mL at 20 °C) -
Is carbon tetrachloride a greenhouse gas?
Yes, it contributes to global warming. -
What are some alternatives to carbon tetrachloride?
Trichloroethylene, methylene chloride, and tetrachloroethylene -
Can carbon tetrachloride be used as a solvent for plastics?
Yes, but only for certain types of plastics. -
Is carbon tetrachloride a flammable substance?
Yes, it has a flash point of -4.3 °C (24.3 °F).
Conclusion
Carbon tetrachloride’s Lewis structure reveals its tetrahedral geometry, nonpolarity, and inertness. Despite its historical uses, its environmental and health risks have led to a decline in its applications. However, understanding its Lewis structure remains essential for chemists studying organic chemistry and environmental science. By following the tips and tricks, avoiding common mistakes, and considering the safety concerns, researchers and practitioners can effectively harness the information provided by this guide.