Introduction
Carbon disulfide (CS2) is a versatile organic compound widely employed in numerous industrial applications. Understanding its molecular shape and properties is crucial for optimizing its use and exploring novel applications. This comprehensive article delves into the intricacies of CS2’s molecular structure, examining its geometry, bond angles, and the implications of its unique shape for its physical and chemical behavior.

Molecular Geometry and Bond Angles
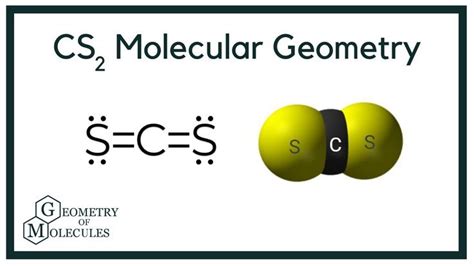
CS2 adopts a linear molecular geometry, with a carbon atom centrally bonded to two sulfur atoms. The carbon-sulfur (C-S) bond length is 1.557 Å, and the S-C-S bond angle is 180 degrees. This linear configuration arises from the sp hybridization of the carbon atom, which results in two unhybridized p orbitals perpendicular to each other. These p orbitals overlap with the p orbitals of the sulfur atoms, forming two sigma bonds.
Implications of the Linear Shape
The linear shape of CS2 has several significant implications for its properties:
-
Low Polarity: The symmetrical distribution of electrons in the molecule results in a low polarity. CS2 has a dipole moment of 0 Debye, making it a nonpolar molecule.
-
High Reactivity: The linear shape allows for efficient overlap of p orbitals, facilitating the formation of covalent bonds. This high reactivity makes CS2 a versatile reagent in chemical reactions.
-
Optical Properties: The linear geometry of CS2 leads to a specific arrangement of energy levels within the molecule. This arrangement influences the molecule’s absorption and emission spectra, giving rise to its characteristic optical properties.
Applications of CS2
The unique molecular shape of CS2 enables its use in a wide range of applications, including:
-
Solvent: CS2’s nonpolar nature makes it an effective solvent for nonpolar compounds, such as oils and greases. It is commonly utilized in the rubber industry and as a starting material for the production of other chemicals.
-
Rayon Production: CS2 is used in the manufacture of rayon, a synthetic fiber derived from cellulose. It is employed as a solvent for the cellulose xanthate solution, which is then extruded into fibers.
-
Fumigation: CS2 acts as a fumigant, used to control pests in grain storage and other agricultural settings. Its vapors penetrate into crevices and kill insects, protecting crops and stored products.
-
Spectroscopy: CS2 exhibits distinctive spectroscopic features. It is used as a reference compound in Raman spectroscopy and as a calibration standard in infrared spectroscopy.
Future Applications
Ongoing research is exploring novel applications for CS2, driven by advancements in nanotechnology and the development of new materials. Potential applications include:
-
Carbon Nanotube Synthesis: CS2 can be used as a precursor in the synthesis of carbon nanotubes, a promising material for electronics and energy storage applications.
-
Graphene Oxide Production: CS2 is investigated as a reagent in the production of graphene oxide, a derivative of graphene with unique electrochemical and optical properties.
-
Antibacterial Coatings: CS2-based coatings are being developed to inhibit bacterial growth on surfaces, offering potential applications in healthcare and hygiene.
Conclusion
The molecular shape of CS2, characterized by its linear geometry and S-C-S bond angle of 180 degrees, has profound implications for its properties and applications. Understanding these properties enables scientists and engineers to optimize the use of CS2 in existing applications and explore novel avenues for its utilization. As research continues to uncover new possibilities, the unique molecular shape of CS2 remains a cornerstone in the exploration of innovative materials and technologies.
Tables
Table 1: Molecular Properties of CS2
| Property | Value |
|---|---|
| Molecular Weight | 76.13 g/mol |
| Density | 1.263 g/cm³ (20 °C) |
| Melting Point | -111.6 °C |
| Boiling Point | 46.26 °C |
| Dipole Moment | 0 Debye |
Table 2: Bond Parameters of CS2
| Bond | Length (Å) | Angle (°) |
|---|---|---|
| C-S | 1.557 | 180 |
Table 3: Applications of CS2
| Application | Description |
|---|---|
| Solvent | Nonpolar solvent for oils and greases |
| Rayon Production | Starting material for cellulose xanthate solution |
| Fumigation | Pest control in grain storage |
| Spectroscopy | Reference compound and calibration standard |
Table 4: Potential Future Applications of CS2
| Application | Description |
|---|---|
| Carbon Nanotube Synthesis | Precursor for carbon nanotube production |
| Graphene Oxide Production | Reagent for graphene oxide synthesis |
| Antibacterial Coatings | Ingredient in coatings to inhibit bacterial growth |