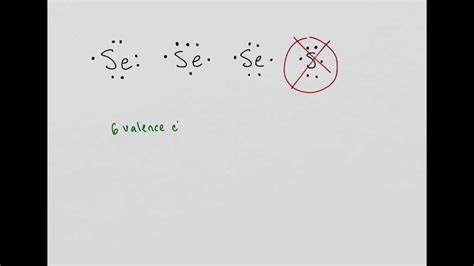Introduction
Selenium is a vital trace element with diverse applications in various industries. Understanding its Lewis dot structure is crucial for comprehending its chemical behavior and predicting its reactivity. This article delves into the fundamental concepts of selenium’s Lewis dot structure, its properties, and its significance.

Lewis Dot Structure
The Lewis dot structure of selenium represents the distribution of its valence electrons. Selenium (Se) has six valence electrons, which are shown as dots surrounding the chemical symbol:
:Se:
The colon (:) represents an electron pair, and the dots represent unpaired electrons. The Lewis dot structure indicates that selenium has four unpaired electrons, making it a reactive element that readily forms covalent bonds.
Key Properties
Selenium exhibits several key properties that are influenced by its Lewis dot structure:
- Atomic Number: 34
- Atomic Mass: 78.96
- Group: 16 (Chalcogens)
- Period: 4
- Valence Electrons: 6
- Electronegativity: 2.55
- Melting Point: 221°C
- Boiling Point: 685°C
- Density: 4.82 g/cm³
Applications of Selenium
Selenium finds wide-ranging applications due to its unique properties:
- Electronics: Used in the production of semiconductors, solar cells, and photoreceptors.
- Glass Industry: Added to glass to reduce its color and improve clarity.
- Health: Used as a dietary supplement to prevent selenium deficiency and support the immune system.
- Antioxidant: Acts as a potent antioxidant, protecting cells from oxidative damage.
- Catalyst: Employed in various industrial processes to accelerate chemical reactions.
New Applications for Selenium
Emerging research is uncovering novel applications for selenium:
- Quantum Computing: Selenium nanocrystals have shown promise as potential qubits in quantum computing devices.
- Nanomedicine: Selenium nanoparticles are being explored for targeted drug delivery and biomedical imaging.
- Memristors: Selenium-based materials exhibit memristor properties, enabling the development of next-generation non-volatile memory devices.
- Renewable Energy: Selenium compounds are being investigated for use in solar energy conversion and hydrogen production.
Tables
Table 1: Physical Properties of Selenium
| Property | Value |
|---|---|
| Atomic Number | 34 |
| Atomic Mass | 78.96 |
| Melting Point | 221°C |
| Boiling Point | 685°C |
| Density | 4.82 g/cm³ |
Table 2: Chemical Properties of Selenium
| Property | Value |
|---|---|
| Electronegativity | 2.55 |
| Valence Electrons | 6 |
| Oxidation States | -2, 0, +2, +4, +6 |
Table 3: Applications of Selenium
| Industry | Application |
|---|---|
| Electronics | Semiconductors, solar cells |
| Glass | Coloring, clarity |
| Health | Dietary supplement, antioxidant |
| Chemical | Catalyst |
Table 4: Emerging Applications of Selenium
| Field | Application |
|---|---|
| Quantum Computing | Qubits |
| Nanomedicine | Drug delivery, imaging |
| Memristors | Non-volatile memory |
| Renewable Energy | Solar conversion, hydrogen production |
Conclusion
The Lewis dot structure of selenium provides a fundamental understanding of its chemical behavior and reactivity. With its unique properties and diverse applications, selenium plays a significant role in various industries, including electronics, healthcare, and renewable energy. Ongoing research continues to uncover novel applications for this versatile element, opening up new possibilities in cutting-edge technologies and innovative solutions.