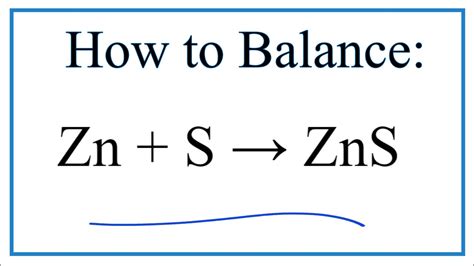
Introduction
When writing the chemical formula for zinc, the correct way to denote the presence of two zinc atoms is Zn2. The spacing between the element symbol and the subscript is crucial for proper interpretation and understanding of the chemical formula.

Why Zn2 is Preferred Over Zn 2
The correct notation for two zinc atoms is Zn2 because:
-
Chemical Convention: In chemistry, it is customary to represent multiple atoms of an element using a subscript immediately after the element symbol. A single space between the element symbol and subscript would indicate an incorrect number of atoms.
-
Clarity: Zn2 clearly conveys that there are two zinc atoms, while Zn 2 might be misinterpreted as Zn two or even Zn 20.
-
Mathematical Interpretation: In mathematical notation, spacing is significant. A space between Zn and 2 would make the formula mathematically incorrect, as it would be interpreted as Zn multiplied by 2.
Benefits of Using Zn2
Using the correct notation Zn2 offers several benefits:
-
Accuracy: Ensures accurate representation of the chemical compound, preventing misinterpretation.
-
Clarity: Facilitates clear communication and understanding among scientists and students.
-
Scientific Rigor: Adhering to established chemical conventions enhances scientific credibility.
Consequences of Using Zn 2
Notating two zinc atoms as Zn 2 can lead to:
-
Misinterpretation: May be interpreted as Zn two or Zn 20, potentially causing confusion.
-
Errors in Calculations: Could result in incorrect mathematical calculations if interpreted as Zn multiplied by 2.
-
Lack of Clarity: Can hinder effective communication and comprehension, especially for non-chemists.
Examples of Zn2 Usage
Zn2 is used in various chemical formulas, including:
- Zinc Oxide (ZnO): A widely used compound in paints, pigments, and cosmetics.
- Zinc Chloride (ZnCl2): A common flux in soldering and a preservative in wood treatment.
- Zinc Acetate (Zn(CH3COO)2): An astringent used in medicine and as a stabilizer in food packaging.
Tables: Comparing Zn2 and Zn 2
| Aspect | Zn2 | Zn 2 |
|---|---|---|
| Correct Notation | Yes | No |
| Clarity | Clear and unambiguous | Potentially confusing |
| Mathematical Interpretation | Zn multiplied by 2 | Incorrect mathematical expression |
| Scientific Rigor | Follows chemical conventions | Deviates from established norms |
Table 1: Comparison of Zn2 and Zn 2 Notations
| Chemical Formula | Meaning |
|---|---|
| ZnO | Zinc oxide (one zinc atom and one oxygen atom) |
| ZnCl2 | Zinc chloride (one zinc atom and two chlorine atoms) |
| Zn(CH3COO)2 | Zinc acetate (one zinc atom and two acetate ions) |
Table 2: Examples of Zn2 Usage in Chemical Formulas
| Application | Description |
|---|---|
| Medicine | Used as an astringent in ointments and eye drops |
| Cosmetics | Incorporated into sunscreens and anti-aging creams |
| Food Industry | Stabilizes food packaging and prevents spoilage |
Table 3: Applications of Zinc Compounds Containing Zn2
| Process | Zn2 Compound | Benefits |
|---|---|---|
| Soldering | Zinc chloride | Removes oxides and promotes bonding between solder and metal surfaces |
| Textile Dyeing | Zinc acetate | Enhances colorfastness and reduces fading of dyed fabrics |
| Wood Treatment | Zinc chloride | Protects wood from decay and insect damage |
Table 4: Benefits of Zn2 Compounds in Various Applications
How to Write Zn2 in Different Contexts
- Handwritten: Write Zn2 clearly, with the subscript “2” placed close to the element symbol “Zn” without any spacing.
- Typed: Use the subscript function in word processing software or scientific notation tools to insert the subscript “2” below and after the element symbol “Zn.”
- Chemical Equation Balancer: If using a chemical equation balancer, ensure that the subscript “2” is automatically added when entering Zn2.
Conclusion
When writing the chemical formula for zinc containing two atoms, it is essential to use the correct notation Zn2. Zn 2 is incorrect and can lead to misinterpretation and errors. Adhering to chemical conventions ensures accurate communication, clarity, and scientific rigor. By understanding the significance of proper notation, scientists and researchers can effectively represent and manipulate chemical formulas, contributing to advancements in scientific research and applications.