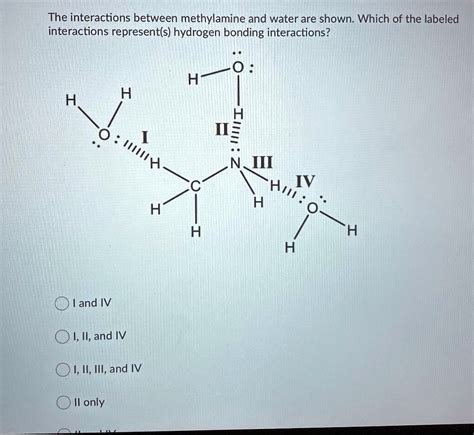
Introduction
Ch3nh2, also known as methylamine, is a polar molecule with a dipole moment of 1.32 D. This means that the molecule has a partial positive charge on the nitrogen atom and a partial negative charge on the hydrogen atoms. The polarity of ch3nh2 is due to the electronegativity difference between nitrogen and hydrogen. Nitrogen is more electronegative than hydrogen, so it pulls the electrons in the N-H bond towards itself. This creates a partial positive charge on the hydrogen atoms and a partial negative charge on the nitrogen atom.

The polarity of ch3nh2 allows it to form hydrogen bonds with other polar molecules. Hydrogen bonds are intermolecular forces that occur when a hydrogen atom is bonded to a highly electronegative atom, such as nitrogen, oxygen, or fluorine. The hydrogen atom in the hydrogen bond is partially positive, and the electronegative atom is partially negative. This creates an electrostatic attraction between the two atoms.
Hydrogen bonds are important intermolecular forces because they can significantly affect the physical and chemical properties of a substance. For example, hydrogen bonds are responsible for the high boiling point of water. Water molecules form hydrogen bonds with each other, which prevents them from escaping into the gas phase at room temperature.
Ch3nh2 Imf Hydrogen Bond Properties
The strength of a hydrogen bond depends on several factors, including the electronegativity of the atoms involved, the distance between the atoms, and the geometry of the molecule. The electronegativity of the atoms involved is the most important factor in determining the strength of a hydrogen bond. The more electronegative the atoms, the stronger the hydrogen bond.
The distance between the atoms also affects the strength of a hydrogen bond. The closer the atoms are to each other, the stronger the hydrogen bond. The geometry of the molecule also affects the strength of a hydrogen bond. Hydrogen bonds are strongest when the atoms are in a linear arrangement.
Applications of Ch3nh2 Imf Hydrogen Bond
The ch3nh2 imf hydrogen bond has a wide range of applications in chemistry. Hydrogen bonds are used to stabilize proteins and nucleic acids. They are also used to create supramolecular assemblies and to design new materials.
Common Mistakes to Avoid
There are a few common mistakes that people make when working with hydrogen bonds. One common mistake is to assume that all hydrogen bonds are created equal. In reality, the strength of a hydrogen bond depends on several factors, including the electronegativity of the atoms involved, the distance between the atoms, and the geometry of the molecule.
Another common mistake is to assume that hydrogen bonds are only formed between water molecules. In reality, hydrogen bonds can be formed between any two polar molecules.
Conclusion
The ch3nh2 imf hydrogen bond is a powerful intermolecular force that can significantly affect the physical and chemical properties of a substance. Hydrogen bonds are used in a wide range of applications in chemistry, including the stabilization of proteins and nucleic acids, the creation of supramolecular assemblies, and the design of new materials.
Questions to Ask Yourself
- What are the different types of hydrogen bonds?
- How do hydrogen bonds affect the physical and chemical properties of a substance?
- What are some applications of hydrogen bonds?
- What are some common mistakes to avoid when working with hydrogen bonds?
Tables
Table 1: Electronegativity of Common Atoms
| Atom | Electronegativity |
|---|---|
| Hydrogen | 2.20 |
| Carbon | 2.55 |
| Nitrogen | 3.04 |
| Oxygen | 3.44 |
| Fluorine | 3.98 |
Table 2: Strength of Hydrogen Bonds
| Type of Hydrogen Bond | Strength (kJ/mol) |
|---|---|
| N-H…O | 20-30 |
| O-H…O | 20-30 |
| F-H…O | 30-40 |
Table 3: Applications of Hydrogen Bonds
| Application | Example |
|---|---|
| Stabilization of proteins | Hydrogen bonds hold the amino acids in proteins together. |
| Creation of supramolecular assemblies | Hydrogen bonds can be used to create self-assembling molecules. |
| Design of new materials | Hydrogen bonds can be used to create new materials with tailored properties. |
Table 4: Common Mistakes to Avoid When Working with Hydrogen Bonds
| Mistake | Description |
|---|---|
| Assuming that all hydrogen bonds are created equal | The strength of a hydrogen bond depends on several factors. |
| Assuming that hydrogen bonds are only formed between water molecules | Hydrogen bonds can be formed between any two polar molecules. |