Introduction
Sulfur dioxide (SF2) is a fascinating inorganic molecule with diverse applications, ranging from industrial processes to environmental monitoring. Understanding its Lewis dot structure is crucial for elucidating its chemical properties and predicting its reactivity. This article provides a comprehensive overview of the Lewis dot structure of SF2, delving into its formation, characteristics, and significance.

The Lewis Dot Structure of SF2
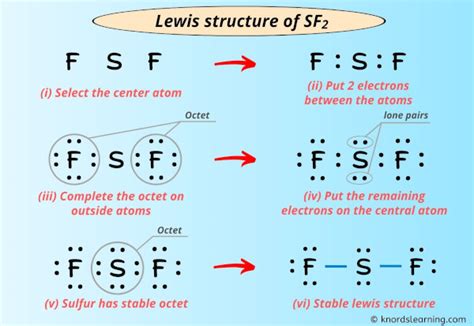
The Lewis dot structure of a molecule depicts the arrangement of valence electrons around its constituent atoms. For SF2, it can be represented as follows:
:F:S:F:
In this structure:
- The sulfur atom (S) is the central atom.
- Each fluorine atom (F) is bonded to the sulfur atom by a single covalent bond.
- The sulfur atom has two lone pairs of electrons.
Formation of the Lewis Dot Structure
To construct the Lewis dot structure of SF2, follow these steps:
- Determine the total number of valence electrons: Sulfur has six valence electrons, while each fluorine atom has seven. Therefore, the total number of valence electrons in SF2 is 20.
- Connect the atoms with single bonds: Draw a line between the sulfur atom and each fluorine atom to represent the single covalent bonds. This accounts for four valence electrons.
- Distribute the remaining valence electrons as lone pairs: The remaining 16 valence electrons are distributed as lone pairs on the fluorine atoms. Each fluorine atom receives three lone pairs, resulting in the structure shown above.
Characteristics of the SF2 Lewis Dot Structure
The Lewis dot structure of SF2 reveals several important characteristics:
- Bent molecular shape: The lone pairs on the fluorine atoms repel each other, causing the molecule to adopt a bent or V-shaped geometry. The F-S-F bond angle is approximately 119.5 degrees.
- Polar covalent bonds: The fluorine atoms are more electronegative than the sulfur atom, resulting in a polar covalent bond between the sulfur and fluorine atoms. This polarity contributes to the molecule’s dipole moment.
- Resonance structures: The sulfur atom can participate in resonance, where the double bond between one of the fluorine atoms and the sulfur atom shifts to the other fluorine atom. This resonance stabilizes the molecule and increases its bond strength.
Significance of the SF2 Lewis Dot Structure
The Lewis dot structure of SF2 is essential for understanding the following aspects:
- Chemical reactivity: The lone pairs on the fluorine atoms make SF2 a Lewis base, capable of donating electron pairs to Lewis acids. This allows SF2 to form coordination complexes with transition metal ions.
- Environmental impact: SF2 is a greenhouse gas that contributes to global warming. Its Lewis dot structure provides insights into its atmospheric behavior and potential abatement strategies.
- Industrial applications: SF2 is used as an etching gas in semiconductor manufacturing, as well as a fumigant in the food industry. Understanding its Lewis dot structure is crucial for optimizing these applications.
Beyond the Lewis Dot Structure: A Look into New Applications
The Lewis dot structure of SF2 serves as a foundation for exploring novel applications in various fields. For example, researchers are investigating the following:
- Sensor technology: The dipole moment of SF2 makes it a potential candidate for use in gas sensors.
- Medical imaging: Sulfur-based compounds, including SF2, are being studied for their potential in medical imaging techniques.
- Energy storage: SF2 is being explored as a potential component in rechargeable batteries.
Tips and Tricks for Understanding Lewis Dot Structures
- Practice regularly: The more you practice drawing Lewis dot structures, the more proficient you will become.
- Use reference materials: Utilize textbooks, online resources, and periodic tables to assist you in determining the valence electrons of different atoms.
- Consider molecular shape: The Lewis dot structure can provide insights into the molecular shape, which influences its properties.
- Remember resonance: For molecules with multiple resonance structures, consider all possibilities to account for the most stable configuration.
Conclusion
The Lewis dot structure of SF2 provides a comprehensive understanding of its chemical properties and behavior. It highlights the molecule’s bent molecular shape, polar covalent bonds, and resonance structures. By exploring the significance and potential applications beyond the Lewis dot structure, we can continue to advance our knowledge and harness the versatility of this fascinating molecule.