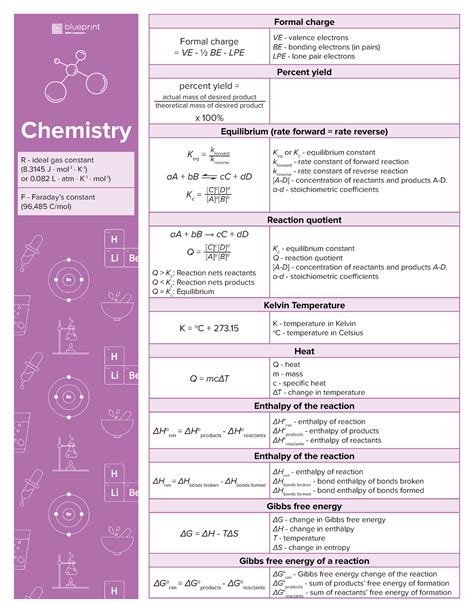
The Medical College Admission Test (MCAT) is a standardized exam that medical school applicants must take. The exam covers a wide range of topics, including chemistry, biology, physics, and psychology. Chemistry is a particularly important section of the MCAT, as it accounts for 25% of the total score.

To do well on the MCAT chemistry section, you need to be familiar with a wide range of chemical equations. These equations can be used to solve problems, make predictions, and explain chemical phenomena.
In this article, we will provide you with a comprehensive guide to MCAT chemistry equations. We will cover everything you need to know about these equations, including their format, how to use them, and how to memorize them.
Format of MCAT Chemistry Equations
MCAT chemistry equations are typically written in the following format:
reactants → products
The reactants are the substances that are present at the beginning of the reaction, and the products are the substances that are formed at the end of the reaction. The arrow indicates the direction of the reaction.
For example, the following equation shows the reaction between hydrogen and oxygen to form water:
2H2 + O2 → 2H2O
In this equation, hydrogen and oxygen are the reactants, and water is the product. The arrow indicates that the reaction proceeds from left to right.
How to Use MCAT Chemistry Equations
MCAT chemistry equations can be used to solve problems, make predictions, and explain chemical phenomena.
Solving Problems
Chemistry equations can be used to solve a variety of problems, such as:
- Calculating the amount of product that will be formed
- Determining the limiting reactant
- Predicting the products of a reaction
For example, the following problem can be solved using the equation for the reaction between hydrogen and oxygen:
How many grams of water will be produced when 10.0 g of hydrogen reacts with 10.0 g of oxygen?
To solve this problem, we can use the following steps:
- Convert the masses of the reactants to moles.
- Use the mole ratio from the equation to determine the number of moles of water that will be produced.
- Convert the number of moles of water to grams.
The solution to this problem is as follows:
- Convert the masses of the reactants to moles.
10.0 g H2 × (1 mol H2 / 2.02 g H2) = 4.95 mol H2
10.0 g O2 × (1 mol O2 / 32.00 g O2) = 0.313 mol O2
- Use the mole ratio from the equation to determine the number of moles of water that will be produced.
4.95 mol H2 × (2 mol H2O / 2 mol H2) = 4.95 mol H2O
- Convert the number of moles of water to grams.
4.95 mol H2O × (18.02 g H2O / 1 mol H2O) = 89.1 g H2O
Therefore, 10.0 g of hydrogen will react with 10.0 g of oxygen to produce 89.1 g of water.
Making Predictions
Chemistry equations can be used to make predictions about the products of a reaction. For example, the following equation shows the reaction between sodium and chlorine to form sodium chloride:
2Na + Cl2 → 2NaCl
This equation tells us that sodium and chlorine will react to form sodium chloride. However, the equation does not tell us anything about the rate of the reaction or the conditions under which the reaction will occur.
To make predictions about the products of a reaction, we need to use our knowledge of chemistry. For example, we know that sodium is a very reactive metal and that chlorine is a very reactive gas. Therefore, we can predict that the reaction between sodium and chlorine will be very rapid and that it will produce a large amount of heat.
Explaining Chemical Phenomena
Chemistry equations can be used to explain chemical phenomena. For example, the following equation shows the reaction between carbon dioxide and water to form carbonic acid:
CO2 + H2O → H2CO3
This equation explains why carbon dioxide dissolved in water produces a