Introduction
Understanding the Lewis structure of N2H4, commonly known as hydrazine, is crucial for comprehending its chemical properties and extensive applications. This article delves into the intricacies of the Lewis structure of hydrazine, exploring its molecular geometry, bonding characteristics, and electronic configuration.

What is Hydrazine?
Hydrazine is a colorless, toxic, and highly reactive inorganic compound with the chemical formula N2H4. It is a weak base and a strong reducing agent. Hydrazine finds applications in various fields, including rocket propulsion, fuel cells, photography, and chemical synthesis.
The Lewis Structure of Hydrazine
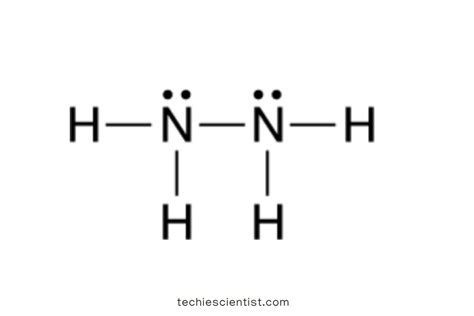
The Lewis structure of a molecule represents the bonding and arrangement of atoms and electrons within the molecule. The Lewis structure of hydrazine can be constructed as follows:
- Count the total number of valence electrons: Nitrogen has five valence electrons, and hydrogen has one valence electron. Therefore, the total number of valence electrons in N2H4 is (2 × 5) + (4 × 1) = 14.
- Connect the nitrogen atoms: The two nitrogen atoms are connected by a single bond, represented as N-N.
- Add the hydrogen atoms: Each nitrogen atom is bonded to two hydrogen atoms, forming four N-H bonds.
- Distribute the remaining electrons: The remaining six electrons are distributed as lone pairs on the nitrogen atoms, resulting in two lone pairs on each nitrogen atom.
Molecular Geometry
The Lewis structure of hydrazine indicates that it has a staggered tetrahedral geometry. This geometry arises from the hybridization of the nitrogen atoms, which possess four electron pairs (two lone pairs and two bonding pairs). The four electron pairs adopt a tetrahedral arrangement around each nitrogen atom, resulting in the staggered tetrahedral molecular geometry.
Bonding in Hydrazine
Hydrazine exhibits covalent bonding, in which electrons are shared between atoms to form chemical bonds. The N-N bond is a single covalent bond, formed by the overlap of one pair of electrons from each nitrogen atom. The N-H bonds are also covalent bonds, formed by the overlap of one pair of electrons from each nitrogen atom and one electron from each hydrogen atom.
Electronic Configuration
The electronic configuration of hydrazine can be described using molecular orbital theory. The molecular orbitals are formed by the combination of the atomic orbitals of the constituent atoms. The molecular orbital diagram of hydrazine shows that the lowest energy molecular orbital is a σ-bonding orbital, followed by a degenerate set of π-bonding orbitals. The highest energy molecular orbitals are a degenerate set of σ*-antibonding orbitals.
Physical and Chemical Properties of Hydrazine
The Lewis structure of hydrazine provides insights into its physical and chemical properties. Some notable properties include:
- Physical State: Colorless, fuming liquid
- Density: 1.008 g/mL
- Boiling Point: 113.5 °C
- Melting Point: 2 °C
- Solubility: Soluble in water, alcohol, and ether
- Reactivity: Highly reactive, strong reducing agent
Applications of Hydrazine
Hydrazine finds applications in numerous industries due to its unique properties. Some key applications include:
- Rocket Propulsion: Hydrazine is used as a fuel for small rockets and spacecraft, providing high thrust and specific impulse.
- Fuel Cells: Hydrazine is employed in fuel cells as a source of energy for portable power applications.
- Photography: Hydrazine is used in photographic developers and flash tubes to enhance the sensitivity and light output.
- Chemical Synthesis: Hydrazine is utilized as a reducing agent in various chemical reactions, particularly in the synthesis of pharmaceuticals and dyes.
Emerging Applications of Hydrazine
The Lewis structure of hydrazine opens up possibilities for novel applications in various fields. Researchers are exploring the use of hydrazine in:
- Hydrogen Storage: Hydrazine can be used as a hydrogen storage medium, releasing hydrogen upon decomposition.
- Pharmaceuticals: Hydrazine derivatives are being investigated for their potential as antibacterial and anticancer agents.
- Materials Science: Hydrazine-based polymers are being developed with enhanced properties for use in aerospace and electronics.
Conclusion
The Lewis structure of N2H4 provides essential information about the bonding, geometry, and electronic configuration of hydrazine. Understanding the Lewis structure is critical for comprehending the properties and applications of this versatile compound. As research continues to uncover new applications for hydrazine, its unique chemistry will continue to drive innovation in various fields.
Tables
Table 1: Physical and Chemical Properties of Hydrazine
| Property | Value |
|---|---|
| Molecular Formula | N2H4 |
| Molecular Weight | 32.05 g/mol |
| Physical State | Colorless liquid |
| Density | 1.008 g/mL |
| Boiling Point | 113.5 °C |
| Melting Point | 2 °C |
| Solubility | Soluble in water, alcohol, and ether |
| Reactivity | Highly reactive, strong reducing agent |
Table 2: Applications of Hydrazine
| Application | Industry |
|---|---|
| Rocket Propulsion | Aerospace |
| Fuel Cells | Energy |
| Photography | Imaging |
| Chemical Synthesis | Pharmaceuticals, Dyes |
Table 3: Emerging Applications of Hydrazine
| Application | Field |
|---|---|
| Hydrogen Storage | Energy |
| Pharmaceuticals | Medicine |
| Materials Science | Aerospace, Electronics |
Table 4: FAQs about Hydrazine
| Question | Answer |
|---|---|
| What is hydrazine used for? | Hydrazine is used in rocket propulsion, fuel cells, photography, and chemical synthesis. |
| Is hydrazine toxic? | Yes, hydrazine is a toxic and corrosive compound. |
| What is the Lewis structure of hydrazine? | The Lewis structure of hydrazine is H2N-NH2. |
| What is the molecular geometry of hydrazine? | Hydrazine has a staggered tetrahedral molecular geometry. |
| What are the bonding characteristics of hydrazine? | Hydrazine exhibits covalent bonding, with a single N-N bond and four N-H bonds. |