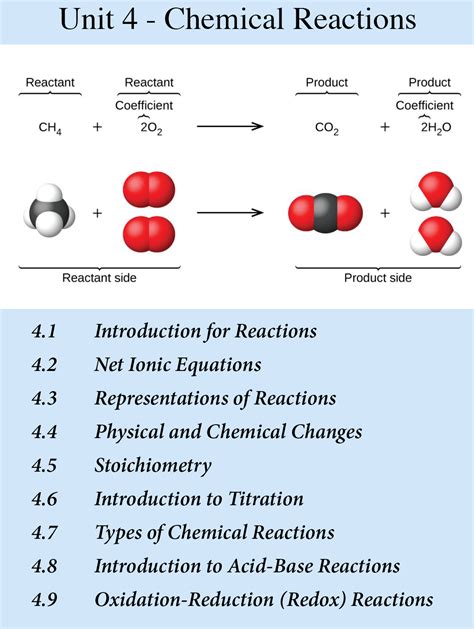
Embark on a captivating journey through Unit 4 of AP Chemistry, where you will unravel the intricate world of chemical equilibrium. This in-depth exploration will empower you to comprehend the dynamic nature of chemical reactions and predict their outcomes with confidence.

Chemical Equilibrium: A Dance of Opposing Forces
Chemical equilibrium occurs when the forward and reverse reactions of a reversible reaction proceed at equal rates, resulting in no net change in the concentrations of the reactants and products. This delicate balance is governed by two laws:
1. Law of Mass Action: The rate of a chemical reaction is directly proportional to the molar concentrations of the reactants.
2. Law of Conservation of Mass: Matter cannot be created or destroyed during a chemical reaction.
Quantitative Equilibrium: Equating the Opposites
To quantify equilibrium, we use the equilibrium constant, denoted by K. K represents the ratio of the molar concentrations of the products to the reactants at equilibrium.
K = [Products] / [Reactants]
K is a constant for a specific reaction at a given temperature. It provides valuable insights into the extent of reaction and the relative amounts of reactants and products at equilibrium.
Factors Influencing Equilibrium
Several factors can shift the equilibrium position of a reaction:
- Temperature: Increasing temperature favors the endothermic reaction (absorbing heat) and shifts the equilibrium towards the products.
- Concentration: Adding more reactants shifts the equilibrium towards the products, while adding more products shifts the equilibrium towards the reactants.
- Pressure (for gases only): Increasing pressure shifts the equilibrium towards the side with fewer moles of gas.
- Catalysts: Catalysts speed up both forward and reverse reactions but do not affect the equilibrium position.
Applications of Chemical Equilibrium
Understanding chemical equilibrium has far-reaching applications in various fields, from industrial processes to biological systems. Here are a few examples:
- Industrial Chemistry: Ammonia synthesis, the Haber process, heavily relies on chemical equilibrium to maximize ammonia production.
- Acid-Base Chemistry: The pH of a solution is determined by the equilibrium between hydrogen ions and hydroxide ions.
- Physiology: The regulation of blood pH involves a delicate balance of chemical equilibria involving carbonic acid and bicarbonate ions.
Common Mistakes to Avoid
To excel in Unit 4 AP Chem, avoid these common mistakes:
- Neglecting the Law of Conservation of Mass
- Misinterpreting the equilibrium constant
- Assuming that reactants are completely consumed at equilibrium
- Ignoring the effects of temperature or concentration on equilibrium
Conclusion
Mastering chemical equilibrium is essential for understanding the dynamic nature of chemical reactions and predicting their outcomes. This knowledge empowers you to delve deeper into complex chemical systems and make informed predictions based on fundamental principles. By embracing the concepts and applications explored in Unit 4 AP Chem, you will gain a profound understanding of the intricate world of chemical reactions.
Useful Tables
| Table | Description |
|---|---|
| Table 1: Equilibrium Constants of Common Reactions | Equilibrium constants for select reactions at 298 K |
| Table 2: Le Chatelier’s Principle | Effects of external changes on equilibrium position |
| Table 3: Acid-Base Equilibrium Constants | Equilibrium constants for acid-base reactions |
| Table 4: Gas Equilibrium Constants | Equilibrium constants for gaseous reactions |
Additional Keywords
- Reversible reactions
- Equilibrium constant
- Le Chatelier’s principle
- Acid-base chemistry
- Industrial applications