Introduction
Bromine trifluoride (BrF3) is a colorless to pale yellow gas with a pungent odor. It is a highly reactive compound that is used in a variety of industrial and laboratory applications. The Lewis structure for BrF3 can provide valuable insights into its molecular geometry, bonding, and properties.

Step-by-Step Approach to Drawing the Lewis Structure for BrF3
- Determine the total number of valence electrons:
Br: 7 valence electrons
F x 3: 3 x 7 = 21 valence electrons
Total: 7 + 21 = 28 valence electrons
- Place the bromine atom in the center of the molecule:
F F F
| | |
Br
- Connect the fluorine atoms to the bromine atom with single bonds:
F F F
| | |
Br-F F
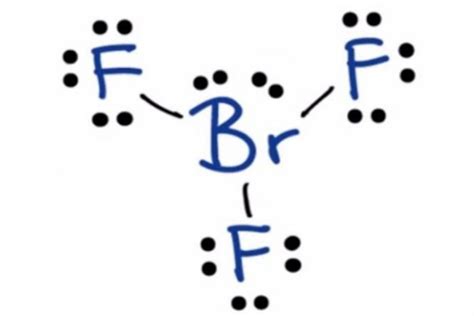
- Distribute the remaining electrons as lone pairs on the fluorine atoms:
F F F
| | |
Br-F F
: : :
: : :
Molecular Geometry and Bonding
The Lewis structure for BrF3 shows that the bromine atom is bonded to three fluorine atoms by single bonds. The three fluorine atoms are arranged in a trigonal pyramidal geometry around the bromine atom. This geometry is consistent with the VSEPR theory, which predicts that the shape of a molecule is determined by the repulsion between its electron pairs.
The Br-F bond length in BrF3 is 1.75 Å, which is shorter than the typical Br-F bond length of 1.84 Å. This indicates that the Br-F bonds in BrF3 are stronger than typical Br-F bonds. The increased bond strength is due to the presence of the lone pairs on the fluorine atoms. The lone pairs donate electrons to the Br-F bonds, which strengthens the bonds.
Properties and Applications
BrF3 is a highly reactive compound that can react with a variety of materials, including metals, non-metals, and organic compounds. It is used in a variety of industrial and laboratory applications, including:
- Rocket fuel: BrF3 is used as an oxidizer in rocket fuel. It is highly reactive and provides a lot of energy when it reacts with fuel.
- Etching agent: BrF3 is used as an etching agent in the semiconductor industry. It is used to remove unwanted material from the surface of semiconductors.
- Fluorination agent: BrF3 is used as a fluorination agent in the pharmaceutical industry. It is used to introduce fluorine atoms into organic molecules.
Tips and Tricks for Drawing Lewis Structures
- Use the periodic table to determine the number of valence electrons for each atom.
- Start by connecting the atoms with single bonds.
- Distribute the remaining electrons as lone pairs on the atoms.
- Check the VSEPR theory to determine the molecular geometry.
- Use formal charges to determine the most stable Lewis structure.
Conclusion
The Lewis structure for BrF3 provides valuable insights into its molecular geometry, bonding, and properties. BrF3 is a highly reactive compound that is used in a variety of industrial and laboratory applications. By understanding the Lewis structure for BrF3, scientists can better understand its properties and develop new applications for this versatile compound.