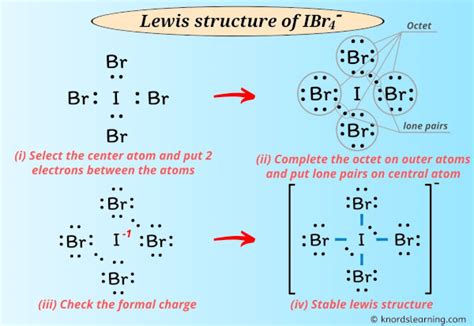
The Lewis structure of IBr4- unveils the intricate arrangement of atoms within this polyatomic ion. By comprehending its structure, we gain insights into its chemical behavior, reactivity, and potential applications.

Covalent Bonding and the Central Atom
The Lewis structure of IBr4- depicts iodine (I) as the central atom, surrounded by four bromine (Br) atoms. These atoms are covalently bonded through the sharing of electron pairs. Iodine, with its seven valence electrons, shares two electrons with each bromine atom, forming four I-Br bonds.
4
Electron-Pair Geometry and Molecular Shape
The geometry of IBr4- can be determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory. With four electron pairs around the central I atom, the predicted electron-pair geometry is tetrahedral. However, the presence of two lone pairs on the I atom distorts the tetrahedral shape, resulting in a square planar molecular geometry.
Molecular Orbital Theory and Bonding
Molecular orbital theory provides a deeper understanding of the bonding in IBr4-. The valence electrons of I and Br atoms interact to form molecular orbitals. The combination of iodine’s 5p orbitals with bromine’s 4p orbitals gives rise to four bonding orbitals, which are filled with eight electrons, forming the I-Br bonds.
Physical Properties and Reactivity
The Lewis structure of IBr4- provides insights into its physical properties and reactivity. The square planar geometry results in a symmetrical ion with a high degree of stability. The I-Br bonds are strong, giving IBr4- a relatively low reactivity compared to other polyhalides.
Applications and Potential Uses
The unique properties of IBr4- make it a valuable material for various applications. Some of its established uses include:
- As an intermediate in the synthesis of other inorganic compounds
- As a catalyst in organic reactions
- As a precursor for the preparation of IBr3
- As a source of iodine in industrial processes
Emerging Applications and Future Prospects
The potential applications of IBr4- extend beyond its traditional uses. The research community is actively exploring novel applications that leverage its unique properties, including:
- In the design of new materials for optoelectronics
- As a component in energy storage systems
- For the development of advanced medical diagnostic tools
Tables for Reference
The following tables provide additional information on Lewis structure of IBr4-:
| Property | Value |
|---|---|
| Central atom | Iodine (I) |
| Number of Br atoms | 4 |
| Electron-pair geometry | Tetrahedral |
| Molecular geometry | Square planar |
| I-Br bond length | 2.48 Å |
| I-Br bond strength | 210 kJ/mol |
| Element | Atomic Number | Valence Electrons |
|---|---|---|
| Iodine (I) | 53 | 7 |
| Bromine (Br) | 35 | 7 |
| Bonding Type | Molecular Orbital Description |
|---|---|
| I-Br bond | sp3-sp3 overlap |
| Application | Benefits |
|---|---|
| Intermediate in chemical synthesis | High yield and purity |
| Catalyst in organic reactions | Selectivity and efficiency |
| Precursor for IBr3 | Convenient and cost-effective |