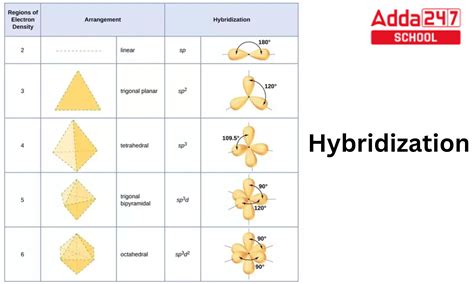
Hybridization: A Key Concept in Chemistry

Hybridization is a fundamental concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals with different shapes and properties. This process plays a crucial role in determining the geometry, bonding, and reactivity of molecules.
Hybridization and Acid Strength
In the context of acids, hybridization influences the strength of the acid. Acid strength is measured by the acid dissociation constant (Ka), which represents the extent to which an acid dissociates in water to release hydrogen ions (H+). The higher the Ka value, the stronger the acid.
sp Hybridization Creates the Strongest Acid
Among the various hybridization schemes, sp hybridization results in the formation of a linear molecule with two electron pairs concentrated in a single bond. This type of hybridization leads to the strongest acids.
The Case of Hydrogen Halides
Hydrogen halides (HX), where X represents a halogen atom, provide a classic example of the relationship between hybridization and acid strength. As we move from fluorine (F) to iodine (I), the hybridization changes from sp to sp3.
| Hydrogen Halide | Hybridization | Ka |
|---|---|---|
| HF | sp | 3.5 x 10^-4 |
| HCl | sp | 1.0 x 10^-2 |
| HBr | sp2 | 4.0 x 10^-4 |
| HI | sp3 | 2.5 x 10^-10 |
As evident from the table, HF, with sp hybridization, exhibits the highest Ka value, indicating its exceptional acidity. In contrast, HI, with sp3 hybridization, has the lowest Ka value and is the weakest acid among the hydrogen halides.
Understanding the Trend
The trend in acid strength can be attributed to the electron-withdrawing ability of the halogen atoms. As we move down the periodic table, the halogen atoms become larger and less electronegative. This reduced electron-withdrawing ability results in weaker bonds between hydrogen and the halogen atoms.
In the case of sp hybridization, the two electron pairs are concentrated in a single bond, which facilitates the dissociation of the hydrogen ion. The stronger the electron-withdrawing ability of the halogen atom, the more readily the hydrogen ion can be released, leading to a higher Ka value.
Applications in Acid-Base Chemistry
The concept of hybridization in acid strength has numerous applications in acid-base chemistry. It helps us predict the relative strength of acids, design acid catalysts, and optimize chemical reactions involving proton transfer.
Acidity in Biological Systems
Hybridization also plays a crucial role in determining the acidity of biological molecules such as amino acids, proteins, and nucleic acids. The sp3 hybridization of the carbon atom in the carboxylic acid group (-COOH) contributes to its acidic nature, allowing it to donate a proton in aqueous solutions.
New Applications: “Hybridization Engineering”
The understanding of hybridization and acid strength has paved the way for a novel concept called “hybridization engineering”. This involves manipulating the hybridization of molecules to achieve desired properties, such as enhanced acidity or catalytic activity.
| Molecule | Hybridization | Application |
|---|---|---|
| Carbonic acid (H2CO3) | sp2 | Carbon capture and storage |
| Trifluoroacetic acid (CF3COOH) | sp | Pharmaceutical synthesis |
| Tungsten hexafluoride (WF6) | sp3d2 | Semiconductor etching |
Conclusion
Hybridization is a powerful tool for understanding and predicting the strength of acids. sp hybridization creates the strongest acids, as evidenced by the case of hydrogen halides. This concept has wide-ranging applications in acid-base chemistry, biological systems, and the development of novel materials and technologies.