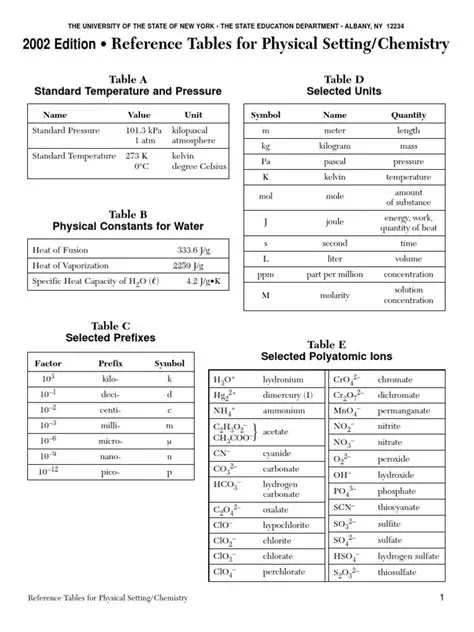
Are you preparing for the Chemistry Regents exam and feeling overwhelmed by all the information you need to remember? Fear not! A reference table is your secret weapon to organize and master essential chemistry concepts.

The Power of Reference Tables
A reference table is a concise summary of important equations, constants, and other data that you can quickly access during the exam. It serves as a valuable tool for:
- Saving Time: Instead of flipping through your textbook or notes, you can easily locate the information you need on your reference table.
- Reducing Stress: Having a reference table at your fingertips can give you the confidence to tackle even the most challenging questions.
- Improving Accuracy: When you have the correct information readily available, you’re less likely to make mistakes.
Core Components of a Chemistry Regents Reference Table
A comprehensive Chemistry Regents reference table should include the following sections:
Equations and Conversions
- Balancing chemical equations
- Stoichiometry calculations (moles, mass, volume)
- Equilibrium expressions
- Gas laws
Physical Constants
- Avogadro’s number
- Universal gas constant
- Faraday constant
- Ideal gas constant
Periodic Trends
- Atomic radius
- Ionization energy
- Electronegativity
- Electron affinity
Acid-Base Properties
- pH calculations
- Titration equations
- Strong and weak acids and bases
Electrochemistry
- Redox reactions
- Half-reactions
- Standard reduction potentials
- Faraday’s law of electrolysis
Crafting Your Own Reference Table
To create an effective reference table, follow these steps:
- Identify Essential Concepts: Review the Chemistry Regents curriculum and pinpoint the key concepts you need to know.
- Choose Relevant Data: Select the equations, constants, and information that are most frequently tested on the exam.
- Organize and Format: Arrange the data in a clear and concise manner, using tables, lists, or flowcharts.
- Practice and Revise: Utilize your reference table regularly and make revisions as needed to improve its accuracy and effectiveness.
Sample Reference Table
Below is a sample reference table that you can use as a starting point for creating your own:
| Section | Information |
|---|---|
| Equations | Balancing equations, stoichiometry, equilibrium, gas laws |
| Physical Constants | Avogadro’s number, gas constant, Faraday constant, ideal gas constant |
| Periodic Trends | Atomic radius, ionization energy, electronegativity, electron affinity |
| Acid-Base Properties | pH calculations, titration equations, acid-base strength |
| Electrochemistry | Redox reactions, half-reactions, reduction potentials, Faraday’s law |
Strategies for Using Your Reference Table
- Become Familiar: Get to know your reference table thoroughly before the exam.
- Use Wisely: Don’t rely on your reference table as a substitute for understanding the concepts.
- Time Your Use: During the exam, take time to find the information you need quickly.
- Check Your Answers: If you’re unsure about an answer, consult your reference table to confirm or correct it.
Conclusion
A well-crafted reference table can significantly enhance your performance on the Chemistry Regents exam. By embracing its power, you can organize essential knowledge, reduce stress, and achieve your academic goals. Remember, a reference table is not a cheat sheet but a tool to empower your success.