Introduction

Formaldehyde, a colorless gas with a pungent odor, is a ubiquitous chemical compound found in various everyday products, from building materials to cosmetics. Understanding its intermolecular forces is crucial for comprehending its behavior and predicting its applications. This article delves into the fascinating realm of formaldehyde intermolecular forces, exploring their nature, effects, and potential implications.
Intermolecular Forces in Formaldehyde
Intermolecular forces are attractive or repulsive interactions between molecules. They govern the physical properties of substances, such as their state of matter, boiling point, and melting point. In the case of formaldehyde, the primary intermolecular force responsible for its behavior is the dipole-dipole interaction.
Dipole-Dipole Interaction
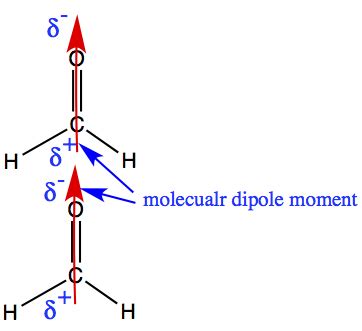
Dipole-dipole interactions arise due to the presence of polar bonds within molecules. A polar bond is formed between two atoms with different electronegativities, causing a shift of electron density towards the more electronegative atom. This results in a partial positive charge on one atom and a partial negative charge on the other, creating a molecular dipole.
Formaldehyde possesses a significant dipole moment due to the presence of the highly electronegative oxygen atom bonded to the less electronegative carbon atom. This dipole creates an electrostatic attraction between neighboring formaldehyde molecules, aligning them in a head-to-tail fashion.
Consequences of Dipole-Dipole Interactions
The dipole-dipole interactions in formaldehyde have several notable consequences:
Liquid Phase
Formaldehyde’s relatively strong dipole-dipole interactions prevent it from readily vaporizing at room temperature. Instead, it exists as a liquid at 20°C, despite its low molecular weight of 30.03 g/mol.
Boiling Point
The boiling point of formaldehyde, 96.8°C, is significantly higher than that of similar-sized nonpolar molecules, such as propane (-42.1°C). This elevated boiling point reflects the energy required to overcome the dipole-dipole interactions and vaporize the liquid.
Melting Point
Formaldehyde’s dipole-dipole interactions also contribute to its relatively high melting point (-92°C). The attractive forces between molecules stabilize the solid phase, making it difficult to melt.
Applications of Dipole-Dipole Interactions
Harnessing formaldehyde’s intermolecular forces has led to various practical applications:
Adhesives and Resins
Formaldehyde’s polar nature allows it to form strong bonds with polar surfaces, making it an effective adhesive in plywood and particleboard manufacturing. It is also used in the production of resins, including urea-formaldehyde resin and phenol-formaldehyde resin, which are employed in adhesives, plastics, and building materials.
Disinfectants
Formaldehyde’s strong dipole-dipole interactions enable it to penetrate and denature proteins, making it an effective disinfectant. It is commonly used in hospitals and laboratories to sterilize surfaces and equipment.
Preservatives
The antimicrobial properties of formaldehyde result from its ability to interact with and damage cellular proteins. This makes it a valuable preservative in food and cosmetic products, inhibiting bacterial and fungal growth.
Future Applications – Futroiding
The unique intermolecular forces of formaldehyde present opportunities for the development of novel applications. Futroiding, the process of envisioning and developing future technologies based on scientific principles, suggests several potential avenues of exploration:
Medical Diagnostics
Formaldehyde’s ability to interact with proteins could be harnessed in diagnostic tests for protein-based diseases, such as Alzheimer’s and Parkinson’s.
Smart Materials
The dipole-dipole interactions in formaldehyde could be exploited to design materials with self-assembling properties or tailored electrical and optical properties.
Nano-Electronics
The precise manipulation of formaldehyde’s intermolecular forces could enable the fabrication of nanoscale electronic devices with enhanced performance and functionality.
Conclusion
Formaldehyde intermolecular forces, primarily dipole-dipole interactions, play a pivotal role in shaping the physical and chemical properties of this versatile compound. These forces govern its liquid phase, elevated boiling point, high melting point, and ability to form strong bonds with polar surfaces. Harnessing these interactions has led to a wide range of applications in adhesives, resins, disinfectants, and preservatives. Futroiding reveals promising avenues for further exploitation of formaldehyde’s intermolecular forces in cutting-edge technologies, paving the way for innovative materials and advancements in healthcare and electronics.