Sodium, a ubiquitous element in our world, plays a crucial role in various biological processes and industrial applications. At the atomic level, sodium’s electron configuration holds the key to understanding its unique properties and potential.

Sodium’s Electron Configuration: A Missing Electron
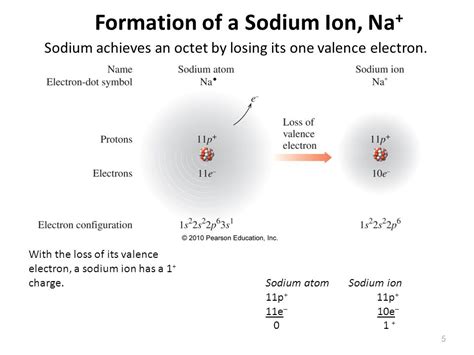
Sodium, with an atomic number of 11, has 11 electrons orbiting its nucleus. In its neutral state, the electronic configuration of sodium is 1s22s22p63s1. This configuration reflects the distribution of electrons across three energy levels or shells.
However, when sodium forms a positive ion (Na+), it loses one electron from its outermost 3s orbital. This results in a stable electron configuration of 1s22s22p6, leaving the ion with a net positive charge.
The Significance of Na+ Electron Configuration
The electron configuration of Na+ has several implications that contribute to its chemical and biological significance:
-
Stability: The loss of an electron results in a noble gas configuration (1s22s22p6), which is energetically favorable. This stability contributes to the ionic nature of Na+ and its low ionization energy.
-
Reactivity: Na+ ions are highly reactive due to the lack of a 3s electron. They readily participate in ionic bonding, forming compounds with negative ions to achieve a stable electron configuration.
-
Biological Functions: Na+ ions play a vital role in maintaining fluid balance and cellular signaling in living organisms. For example, the sodium-potassium pump, located in cell membranes, actively transports Na+ ions out of cells while bringing in potassium ions, creating an electrochemical gradient used for energy-dependent processes.
Applications of Na+: Harnessing the Power of Ions
The unique properties of Na+ ions have led to numerous applications in various fields:
-
Chemical Industry: Na+ is used in the production of sodium hydroxide, sodium carbonate, and other sodium-containing chemicals.
-
Pharmaceuticals: Na+ is essential in the formulation of intravenous fluids and electrolyte solutions used in medical treatments.
-
Food Preservation: Sodium salts, such as sodium chloride (common salt), are widely used as preservatives in the food industry to inhibit microbial growth.
-
Biotechnology and Medicine: Na+ ions are involved in DNA replication, gene expression, and nerve impulse transmission.
Generating Ideas: The “Sodium Catalyst”
The unique properties of Na+ ions and their applications inspire researchers to explore innovative ways to harness their potential. Here’s a creative new word:
- Sodium Catalyst: A concept that aims to leverage the reactivity and ionic nature of Na+ ions to enhance chemical reactions, accelerate biological processes, or develop novel materials.
Tables for Reference
| Characteristic | Sodium | Na+ Ion |
|---|---|---|
| Atomic Number | 11 | 11 |
| Electronic Configuration (Neutral) | 1s22s22p63s1 | 1s22s22p6 |
| Electronic Configuration (Ionic) | N/A | 1s22s22p6 |
| Positive Charge | 0 | +1 |
| Application | Description |
|---|---|
| Chemical Industry | Production of sodium hydroxide, sodium carbonate |
| Pharmaceuticals | Formulation of intravenous fluids, electrolyte solutions |
| Food Preservation | Inhibition of microbial growth |
| Biotechnology and Medicine | DNA replication, gene expression, nerve impulse transmission |
Tips and Tricks for Understanding Na+ Electron Configuration
- Visualize the electron configuration using orbital diagrams or interactive simulations.
- Understand the relationship between electron configuration, ionization energy, and ionic charge.
- Explore the periodic table to identify other elements that form stable positive ions.
How-to Step-by-Step Approach to Understanding Na+ Electron Configuration
- Learn the electronic configuration of sodium in its neutral state (1s22s22p63s1).
- Understand the concept of ionization and how it affects the electron configuration.
- Identify the electron that is lost to form the Na+ ion (3s electron).
- Write the electron configuration of the Na+ ion (1s22s22p6).
FAQs
- Why does sodium form a positive ion? Because it loses an electron from its outermost 3s orbital to achieve a stable noble gas configuration.
- How does Na+ electron configuration affect its reactivity? The lack of a 3s electron makes Na+ highly reactive and prone to ionic bonding.
- What are some examples of applications of Na+? Sodium hydroxide production, food preservation, intravenous fluids, and biotechnology.
- What is a “sodium catalyst”? A theoretical concept that aims to utilize the properties of Na+ ions to enhance chemical reactions or biological processes.
- How can I visualize Na+ electron configuration? Use orbital diagrams or interactive simulations to see the distribution of electrons around the nucleus.
- Why is understanding Na+ electron configuration important? It provides insights into the chemical and biological properties of sodium, as well as its potential applications.