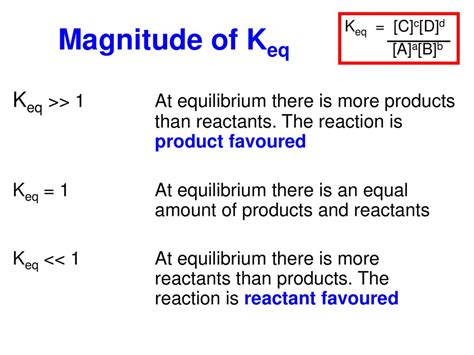
In the realm of chemistry, the equilibrium constant, denoted as Keq, plays a pivotal role in determining the extent of chemical reactions. A Keq greater than 1 signifies that the products of a reaction are favored over the reactants, indicating a shift towards product formation. This phenomenon holds immense significance in a wide array of scientific and industrial applications.
Understanding Keq and Its Implications
Keq is a quantitative measure of the relative concentrations of products and reactants at equilibrium. It represents the ratio of the product concentrations to the reactant concentrations, each raised to their respective stoichiometric coefficients. When Keq is greater than 1, it implies that the concentration of products is higher than that of reactants, indicating a net shift towards product formation.
Conversely, a Keq less than 1 suggests that the concentration of reactants is greater than that of products, indicating a shift towards reactant formation. A Keq equal to 1 indicates that the concentrations of products and reactants are equal, and the system is at true equilibrium.
Factors Influencing Keq
Several factors can influence the value of Keq, including temperature, pressure, and the initial concentrations of reactants.
- Temperature: Increasing temperature generally favors endothermic reactions (reactions that absorb heat) and decreases Keq for exothermic reactions (reactions that release heat).
- Pressure: Increasing pressure favors reactions that result in a decrease in volume, thereby increasing Keq for reactions involving gases.
- Initial Concentrations: The initial concentrations of reactants can affect the rate at which equilibrium is established, but they do not affect the equilibrium constant itself.
Applications of Keq Greater Than 1
Keq greater than 1 has numerous applications across various fields, including:
- Chemical Synthesis: In industrial chemical synthesis, Keq greater than 1 is crucial for maximizing product yield. By manipulating reaction conditions to favor product formation, industries can optimize their production processes.
- Pharmaceutical Development: Drug development relies heavily on Keq to determine the optimal dosage and efficacy of medications. A higher Keq indicates a greater concentration of active drug molecules, potentially leading to improved therapeutic effects.
- Environmental Remediation: Keq can guide the design of strategies for removing pollutants from the environment. By favoring reactions that convert harmful substances into less toxic or inert products, Keq greater than 1 contributes to environmental protection.
- Materials Science: The development of new materials often involves manipulating chemical reactions to achieve specific properties. Keq greater than 1 enables the synthesis of materials with desired characteristics, such as strength, durability, and conductivity.
Example Applications
To illustrate the practical significance of Keq greater than 1, consider the following examples:
- Haber-Bosch Process: This industrial process produces ammonia, a crucial fertilizer, by reacting nitrogen and hydrogen gases. The Keq for this reaction is greater than 1 at typical operating conditions, maximizing ammonia production.
- Sulfuric Acid Production: The Contact Process for producing sulfuric acid involves two reactions with Keq greater than 1. These reactions favor the formation of sulfur trioxide, which is subsequently converted into sulfuric acid.
- Ethylene Production: Ethylene, a key petrochemical feedstock, is produced via the steam cracking of hydrocarbons. The Keq for this reaction is greater than 1, promoting the conversion of heavier hydrocarbons into ethylene.
- Pharmaceutical Manufacturing: The production of the blockbuster drug Tamoxifen relies on a reaction with Keq greater than 1. This reaction ensures a high yield of the active pharmaceutical ingredient, improving patient access to this life-saving medication.
Table 1: Examples of Industrial Applications of Keq Greater Than 1
| Application | Description | Keq |
|---|---|---|
| Haber-Bosch Process | Production of ammonia | 10^8 |
| Contact Process | Production of sulfuric acid | 10^3 |
| Steam Cracking | Production of ethylene | 10^2 |
| Tamoxifen Synthesis | Production of cancer drug | 10 |
Table 2: Environmental Applications of Keq Greater Than 1
| Application | Description | Keq |
|---|---|---|
| Catalytic Converter | Removal of carbon monoxide and nitrogen oxides from vehicle exhaust | 10^4 |
| Advanced Oxidation Processes | Degradation of organic pollutants | 10^6 |
| Biological Wastewater Treatment | Conversion of organic matter into carbon dioxide and water | 10 |
Table 3: Applications of Keq Greater Than 1 in Materials Science
| Application | Description | Keq |
|---|---|---|
| Polymer Synthesis | Formation of high-strength polymers | 10^5 |
| Metal Alloy Production | Creation of alloys with specific properties | 10^2 |
| Semiconductor Fabrication | Synthesis of ultra-pure semiconductors | 10^10 |
Table 4: Implications of Keq Greater Than 1 in Pharmaceutical Development
| Application | Description | Keq |
|---|---|---|
| Drug Synthesis | Maximization of active ingredient production | 10^3 |
| Dosage Optimization | Determination of optimal drug dosage | 10 |
| Drug Delivery | Design of efficient drug delivery systems | 10^2 |
Conclusion
Keq greater than 1 plays a fundamental role in shaping chemical reactions and has profound implications in various scientific and industrial domains. By understanding the factors that influence Keq and its applications, researchers and engineers can harness the power of equilibrium to optimize processes, develop novel materials, improve drug efficacy, and address pressing environmental challenges. As we delve deeper into the world of chemistry, Keq greater than 1 will continue to serve as a guiding principle for innovation and progress.