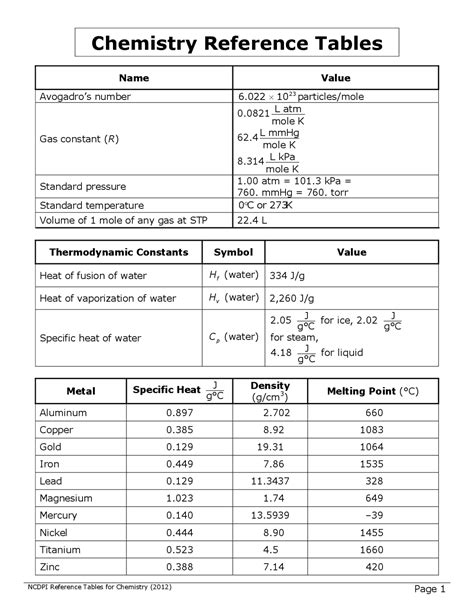
The AP Chemistry Reference Table serves as an indispensable resource for students preparing for the Advanced Placement Chemistry exam. It provides critical data and equations that assist in solving complex chemistry problems and strengthening conceptual understanding.

Importance of the Reference Table
- Time Saver: The reference table eliminates the need to memorize numerous constants and equations, freeing up valuable exam time.
- Problem Solving Tool: It provides essential information for solving equilibrium, stoichiometry, and thermodynamics problems.
- Exam Preparation Guide: The table aligns with the College Board’s AP Chemistry curriculum, covering all major topics tested on the exam.
Updates for 2025
The 2025 AP Chemistry Reference Table has been updated to reflect the latest changes in the exam content. Key modifications include:
- Revised values for gas constant and Faraday’s constant
- Updated bond energies and ionization energies
- Clarifications on atomic radii
- Expanded table of standard reduction potentials
Navigating the Reference Table
The table is organized into several sections:
- Constants and Unit Conversions: Common constants and conversion factors
- Thermochemistry: Enthalpy and entropy values
- Equilibria: Equilibrium constants, acid dissociation constants, and solubility products
- Electrochemistry: Standard reduction potentials, electrode potentials, and cell potentials
- Spectroscopy: Wavelengths and energy levels
- Atomic Structure: Atomic radii, electronegativities, and ionization energies
Effective Strategies for Utilizing the Reference Table
- Familiarize Yourself: Study the table thoroughly before the exam to become comfortable with its contents.
- Mark Important Values: Highlight or circle key constants and equations for quick reference during the exam.
- Practice Application: Solve practice problems using the reference table to enhance problem-solving skills.
- Collaborate with Others: Discuss the reference table with classmates or teachers to gain diverse perspectives.
Common Mistakes to Avoid
- Assuming Precision: Remember that the reference table provides approximate values.
- Ignoring Units: Pay close attention to the units of measure, as they are crucial for accurate calculations.
- Misinterpreting Information: Ensure you fully understand the context and application of the information provided in the table.
Applications of the Reference Table in Chemistry
Beyond the AP Chemistry exam, the reference table has applications in various chemistry-related fields, including:
- Industrial Chemistry: Optimizing chemical reactions for efficiency and cost-effectiveness
- Environmental Science: Understanding chemical reactions in environmental processes
- Medicine: Developing new drugs and treatments based on chemical principles
- Materials Science: Designing advanced materials with tailored properties
Useful Tables for AP Chemistry
In addition to the official AP Chemistry Reference Table, other useful tables exist for students:
- Periodic Table of the Elements: Provides information on atomic number, mass, and electron configuration
- Solubility Table: Lists the solubility of common ionic compounds in water
- Table of Bond Dissociation Energies: Shows the energy required to break various types of bonds
- Table of Standard Enthalpies of Formation: Provides enthalpies of formation for common compounds
Conclusion
The AP Chemistry Reference Table 2025 is a valuable tool that supports students in mastering chemistry concepts and achieving success on the AP exam. By utilizing the reference table effectively, students can optimize their exam preparation, deepen their understanding of chemistry, and prepare for future applications in STEM fields.