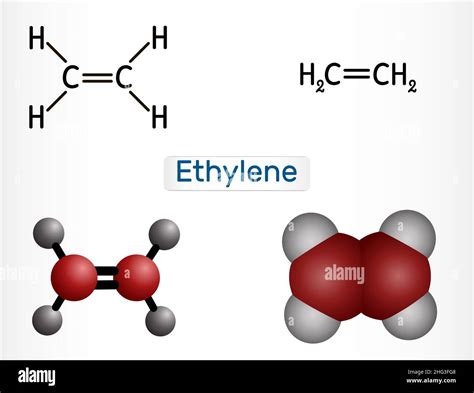
Ethylene, scientifically known as C2H4, is a fundamental organic compound consisting of two carbon atoms and four hydrogen atoms. Its molecular geometry plays a crucial role in determining its chemical properties and reactivity. In this comprehensive article, we will delve into the molecular geometry of C2H4, exploring its shape, bond lengths, and angles.

Hybridization of C2H4
Ethylene’s carbon atoms undergo sp2 hybridization, wherein they form three equivalent sp2 hybrid orbitals that lie in a plane with 120° angles between them. Each carbon atom bonds to two hydrogen atoms via sp2-H sigma bonds, using its three hybrid orbitals. The remaining unhybridized p orbital on each carbon atom overlaps laterally, forming a pi bond between the two carbon atoms.
Molecular Shape of C2H4
The molecular shape of C2H4 is planar, meaning that all atoms lie within the same plane. This planar structure is a consequence of the sp2 hybridization of the carbon atoms. The hydrogen atoms form a plane perpendicular to the carbon-carbon bond, resulting in an overall trigonal planar geometry.
Bond Lengths and Angles
The C-C bond length in C2H4 is 133.9 pm, indicating a double bond between the carbon atoms. The C-H bond lengths are 108.7 pm, signifying the presence of single bonds between the carbon and hydrogen atoms. The bond angles are all approximately 120°, consistent with the sp2 hybridization of the carbon atoms.
| Bond | Bond Length (pm) | Bond Angle (°) |
|---|---|---|
| C-C | 133.9 | 120 |
| C-H | 108.7 | 120 |
Consequences of Molecular Geometry
The planar molecular geometry of C2H4 has significant implications for its properties and reactivity:
- Reactivity: The double bond between the carbon atoms makes C2H4 highly reactive, readily undergoing addition and oxidation reactions.
- Polarity: Despite its overall neutral charge, C2H4 exhibits a slight polarity due to the uneven distribution of electrons between the carbon and hydrogen atoms.
- Intermolecular Forces: The planar shape of C2H4 hinders intermolecular interactions, resulting in weak van der Waals forces between molecules. Consequently, C2H4 is a gas at room temperature.
Applications of C2H4
Ethylene has extensive industrial applications, particularly in the production of plastics and polymers:
- Polyethylene (PE): C2H4 is the primary raw material for the production of polyethylene, one of the most widely used plastics worldwide.
- Polyvinyl chloride (PVC): Ethylene is also used in the manufacture of polyvinyl chloride, a versatile plastic known for its durability and resistance to chemicals.
- Ethylene oxide: C2H4 is converted into ethylene oxide, which serves as a precursor for the production of various chemicals, including antifreeze and detergents.
Innovative Applications
Recent advancements in research have spurred the development of novel applications for C2H4:
- Biofuels: Ethylene can be used as a feedstock for the production of biofuels, such as ethanol, which offer sustainable alternatives to fossil fuels.
- Graphene synthesis: Ethylene serves as a precursor for the synthesis of graphene, an atomically thin material with exceptional electrical and thermal properties.
- Drug delivery: Ethylene-based polymers are employed in the development of drug delivery systems that can release drugs in a controlled manner.
Useful Tables
Table 1: Properties of C2H4
| Property | Value |
|---|---|
| Melting Point | -169.2 °C |
| Boiling Point | -103.9 °C |
| Density | 0.621 g/cm³ (gas) |
| Polarity | Slightly polar |
| Intermolecular Forces | Weak van der Waals forces |
Table 2: Applications of C2H4
| Application | Description |
|---|---|
| Polyethylene (PE) | Widely used plastic with excellent durability and low cost |
| Polyvinyl chloride (PVC) | Versatile plastic resistant to chemicals and weather |
| Ethylene oxide | Precursor for the production of antifreeze and detergents |
| Biofuels | Sustainable alternatives to fossil fuels, such as ethanol |
| Graphene synthesis | Precursor for the synthesis of graphene, a revolutionary material |
| Drug delivery | Development of controlled drug release systems |
Effective Usage in Various Industries
C2H4 is utilized in numerous industries, including:
- Chemical industry: Raw material for the production of various chemicals and polymers
- Plastics industry: Primary feedstock for the production of polyethylene and polyvinyl chloride
- Pharmaceutical industry: Precursor for the synthesis of pharmaceuticals and drug delivery systems
- Energy sector: Feedstock for the production of biofuels
- Materials science: Precursor for the synthesis of graphene and other advanced materials
Comparison of Advantages and Disadvantages
Advantages:
- High reactivity, enabling versatile chemical reactions
- Planar structure, which facilitates intermolecular interactions
- Wide range of applications, from plastics to biofuels
- Abundant availability as a natural gas constituent
Disadvantages:
- Flammability, requiring careful handling
- Potential health hazards associated with inhalation
- Limited solubility in water, which can hinder applications in aqueous systems
Frequently Asked Questions (FAQs)
Q1: What is the hybridization of the carbon atoms in C2H4?
A1: sp2
Q2: What is the molecular shape of C2H4?
A2: Trigonal planar
Q3: What are the bond lengths and angles in C2H4?
A3: C-C: 133.9 pm, 120°; C-H: 108.7 pm, 120°
Q4: What are the major applications of C2H4?
A4: Production of polyethylene, polyvinyl chloride, ethylene oxide, biofuels, graphene, and drug delivery systems
Q5: What safety precautions should be taken when handling C2H4?
A5: Avoid inhalation, use proper ventilation, and handle flammable materials with care.
Q6: How is C2H4 produced industrially?
A6: Primarily through the steam cracking of hydrocarbons
Q7: What is the significance of C2H4 in the petrochemical industry?
A7: Ethylene serves as the building block for numerous chemicals and polymers, forming the foundation of the modern petrochemical industry.
Q8: What are the potential future applications of C2H4?
A8: Continued research is exploring C2H4’s potential in fields such as advanced materials, energy storage, and biotechnology.