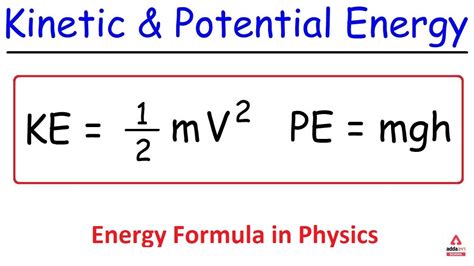
The field of chemistry revolves around energy transformations, making it essential to understand the underlying principles that govern these processes. Energy formula chem provides a powerful framework to calculate the energy changes associated with chemical reactions, enabling chemists to predict and optimize various chemical processes.

The Basics of Energy Formula Chem
At the heart of energy formula chem lies the concept of enthalpy, which measures the total energy content of a system. A chemical reaction involves changes in enthalpy, which can be calculated using the following equation:
ΔH = H(products) - H(reactants)
where:
- ΔH is the change in enthalpy
- H(products) is the enthalpy of the products
- H(reactants) is the enthalpy of the reactants
Thermodynamics and Energy Formula Chem
Thermodynamics plays a crucial role in energy formula chem, providing the laws that govern energy transformations. The first law of thermodynamics states that energy cannot be created or destroyed, only transferred or transformed. In other words, the total energy of a closed system remains constant.
The second law of thermodynamics introduces the concept of entropy, which measures the degree of disorder in a system. This law states that the entropy of an isolated system always increases over time, leading to a decrease in the system’s ability to do work.
Applications of Energy Formula Chem
Energy formula chem finds widespread applications across various fields, including:
- Chemical synthesis: Predict and optimize reaction conditions, maximizing yield and efficiency.
- Material science: Design new materials with desired properties by controlling the energy changes involved in their fabrication.
- Environmental science: Quantify energy changes in environmental processes, such as combustion and pollution control.
- Biochemistry: Understand and manipulate biological processes by analyzing the energy transformations that occur in living organisms.
Common Mistakes to Avoid
To ensure accurate results when using energy formula chem, it’s essential to avoid common mistakes:
- Neglecting the sign of ΔH: A positive ΔH indicates an endothermic reaction (energy absorbed), while a negative ΔH indicates an exothermic reaction (energy released).
- Using incorrect units: Enthalpy is typically expressed in kilojoules per mole (kJ/mol).
- Oversimplifying reactions: Complex reactions may require considering multiple energy changes, such as bond breaking and formation.
Step-by-Step Approach
Applying energy formula chem involves the following steps:
- Identify the reactants and products: Clearly define the initial and final states of the reaction.
- Determine the enthalpy changes: Use thermodynamic tables or equations to find the enthalpy of the reactants and products.
- Calculate the change in enthalpy (ΔH): Subtract the enthalpy of the reactants from the enthalpy of the products.
- Interpret the results: Based on the sign of ΔH, determine whether the reaction is endothermic or exothermic.
Table 1: Enthalpy of Common Substances
| Substance | Enthalpy (kJ/mol) |
|---|---|
| H2O (liquid) | -285.8 |
| CO2 (gas) | -393.5 |
| CH4 (gas) | -74.8 |
| NH3 (gas) | -46.2 |
| NaCl (solid) | -411.1 |
Table 2: Examples of Energy Formulas
| Reaction | Energy Formula |
|---|---|
| Combustion of methane | ΔH = -890.4 kJ/mol |
| Formation of water from hydrogen and oxygen | ΔH = -285.8 kJ/mol |
| Neutralization of hydrochloric acid and sodium hydroxide | ΔH = -57.3 kJ/mol |
| Dissolution of potassium chloride in water | ΔH = +17.4 kJ/mol |
“Energydermis”: A New Word for Innovative Applications
To drive innovation in energy formula chem, we introduce the term “energydermis.” This concept refers to the interface between energy science and other disciplines, creating novel applications:
- Energydermis in medicine: Design new drug molecules with controlled energy release profiles, enhancing drug delivery and efficacy.
- Energydermis in nanotechnology: Develop nanomaterials with tailored energy properties, enabling applications in sensors, electronics, and energy storage.
- Energydermis in sustainability: Create sustainable energy systems by optimizing the energy efficiency of chemical processes and developing renewable energy technologies.
Table 3: Potential Applications of Energydermis
| Field | Example Applications |
|---|---|
| Healthcare | Energy-releasing implants for controlled drug delivery |
| Manufacturing | Energy-efficient chemical synthesis processes |
| Electronics | Nanomaterials with enhanced energy storage capacity |
| Environmental science | Carbon capture technologies using energydermis-based materials |
Table 4: Future Directions for Energy Formula Chem
| Direction | Potential Impact |
|---|---|
| Quantum energy formula chem | Accurate prediction of energy changes at the nanoscale |
| Artificial intelligence-aided energy formula chem | Accelerated development of new chemical processes and materials |
| Interdisciplinary collaboration | Integration of energy formula chem with other fields, such as biology and materials science |
| Sustainable energy formula chem | Development of energy-efficient and environmentally friendly chemical processes |
Conclusion
Energy formula chem provides a powerful tool for understanding and manipulating chemical reactions, enabling advancements in various fields. By embracing the concept of energydermis, researchers can unlock groundbreaking applications, pushing the boundaries of science and technology. As we delve deeper into the complexities of chemical energy, we anticipate further breakthroughs that will shape the future of our world.