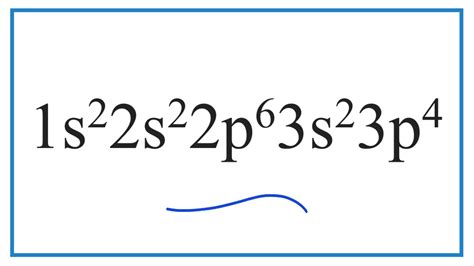Introduction
Nitrogen, with the atomic number 7, is an essential element that plays a crucial role in various biological processes. Its unique electronic configuration, 1s2 2s2 2p6 3s2 3p4, grants it remarkable properties that enable its participation in a wide range of applications.
Electronic Configuration
The electronic configuration of nitrogen describes the arrangement of its electrons in its atomic orbitals. It consists of four energy levels or shells:
- 1s: 2 electrons
- 2s: 2 electrons
- 2p: 6 electrons (3 pairs)
- 3s: 2 electrons
The 3p orbital contains four electrons, which distinguishes nitrogen from carbon and oxygen, its neighboring elements in the periodic table.
Valence Electrons and Bonding
Valence electrons, located in the outermost energy level, determine an element’s chemical reactivity. Nitrogen has three valence electrons (2 in 2p and 1 in 3s). This arrangement allows it to form both covalent and ionic bonds.
- Covalent Bonds: Nitrogen can share its valence electrons with other atoms, forming covalent bonds. For example, nitrogen forms triple bonds with hydrogen to create ammonia (NH3).
- Ionic Bonds: Nitrogen can also lose or gain an electron to achieve a stable octet configuration. By losing three valence electrons, it gains a positive charge (+3) and forms ionic bonds with elements like oxygen in compounds like nitrogen dioxide (NO2).
Applications of Nitrogen
Nitrogen’s unique electronic configuration and reactivity contribute to its diverse applications:
- Fertilizers: Nitrogen is essential for plant growth. Fertilizers containing nitrogen compounds provide the nutrients necessary for plant development.
- Explosives: Nitrogen-containing compounds, such as ammonium nitrate, are used as explosives in construction and mining.
- Pharmaceuticals: Nitrogen is found in many pharmaceutical drugs, including antibiotics, pain relievers, and antidepressants.
- Dyes and Pigments: Nitrogen compounds are used to create a wide range of dyes and pigments for textiles, plastics, and other materials.
Innovative Applications: “Nitegenesis”
Harnessing the electronic configuration of nitrogen holds the potential for groundbreaking applications:
- Nitegenesis: A hypothetical process that could artificially create nitrogen from other elements, addressing the global nitrogen shortage.
- Nitrogen-based Energy Storage: Nitrogen compounds have the potential to store and release energy in high-energy batteries and fuel cells.
- Advanced Materials: Nitrogen-based materials could exhibit unique properties for applications in electronics, semiconductors, and catalysis.
Tables
| Property | Value |
|---|---|
| Atomic Number | 7 |
| Electron Configuration | 1s2 2s2 2p6 3s2 3p4 |
| Valence Electrons | 3 |
| Electronegativity | 3.04 |
| Atomic Radius (pm) | 70 |
| Nitrogen Compound | Application |
|---|---|
| Ammonia (NH3) | Fertilizer, refrigerant |
| Nitric Acid (HNO3) | Fertilizer, explosives |
| Nitrogen Dioxide (NO2) | Air pollutant |
| Cyanide (CN-) | Industrial chemicals, pharmaceuticals |
| Common Mistake to Avoid | Tip |
|---|---|
| Overestimating Nitrogen’s Reactivity | Remember its relatively low electronegativity |
| Underestimating the Importance of Nitrogen in Biology | Recognize its essential role in DNA, proteins, and enzymes |
| Neglecting Nitrogen’s Industrial Applications | Explore its wide range of uses in fertilizers, explosives, and materials |
Conclusion
The electronic configuration of 1s2 2s2 2p6 3s2 3p4 provides nitrogen with unique properties that enable its diverse applications. From fertilizers and pharmaceuticals to advanced materials, nitrogen plays an indispensable role in various industries. Exploring the potential of nitrogen-based innovation, such as “nitegenesis,” holds exciting prospects for solving global challenges and unlocking new technological advancements.