Introduction

Glucose, with the molecular formula C6H12O6, is an essential carbohydrate that serves as the body’s primary energy source. Understanding its molecular structure is crucial for comprehending its biological significance and potential applications. This article delves into the nature of glucose’s chemical bonds, elucidating whether it is ionic or covalent.
Chemical Bonding: A Primer
Chemical bonds arise when atoms exchange or share electrons to achieve a stable configuration. Ionic bonds involve the complete transfer of electrons from one atom to another, resulting in the formation of oppositely charged ions. Covalent bonds, on the other hand, occur when atoms share electrons, forming a molecular orbital.
Glucose’s Molecular Structure
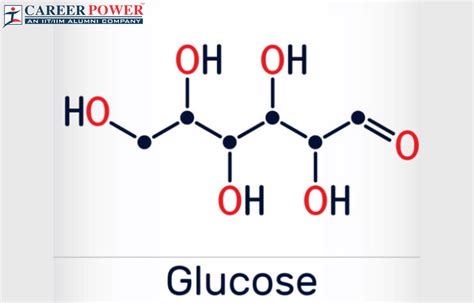
Glucose comprises six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. Its molecular structure is depicted by the following Lewis structure:
H |
O=C-C-C-C-C-O | H
H |
OH |
H
Ionic or Covalent?
Examining the electronegativity of glucose’s constituent atoms reveals that carbon and oxygen differ significantly in their ability to attract electrons. Oxygen has a higher electronegativity than carbon, meaning it has a stronger pull towards shared electrons.
However, the carbon-oxygen bonds in glucose are not typically considered fully ionic, where electrons are completely transferred from carbon to oxygen. Instead, the electrons are shared to a certain extent, indicating a degree of covalent character.
Polar Covalent Bonds and Resonance
To fully understand glucose’s bonding nature, the concept of polar covalent bonds and resonance must be introduced. Polar covalent bonds arise when electrons are unequally shared between two atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other.
In glucose, the carbon-oxygen bonds exhibit polar covalent character due to the difference in electronegativity between carbon and oxygen. Furthermore, glucose’s molecular structure allows for resonance, where the double bond between one carbon and oxygen atom can shift to another carbon-oxygen pair, resulting in multiple resonance structures.
Table 1: Electronegativity of Glucose’s Constituent Atoms
| Element | Electronegativity |
|---|---|
| Carbon | 2.55 |
| Oxygen | 3.44 |
| Hydrogen | 2.20 |
Table 2: Bond Type in Glucose
| Bond Type | Presence |
|---|---|
| Ionic | Absent |
| Covalent | Present |
| Polar Covalent | Present |
| Nonpolar Covalent | Absent |
Implications of Glucose’s Molecular Structure
Glucose’s covalent nature has profound implications for its biological functions and potential applications:
- Energy Storage and Release: The covalent bonds in glucose store energy that can be released through cellular respiration, providing energy for various cellular processes.
- Biomolecule Interactions: The partial positive and negative charges on glucose’s polar covalent bonds allow it to interact with other biomolecules, such as proteins and enzymes.
- Pharmaceutical Applications: Understanding the covalent nature of glucose can aid in the design of drugs that target specific glucose receptors or enzymes involved in glucose metabolism.
- Food Science: The polar covalent character of glucose influences its solubility and other properties crucial for food processing and product development.
Common Mistakes to Avoid
- Mistaking glucose’s polar covalent bonds for ionic bonds.
- Oversimplifying the molecular structure of glucose by ignoring resonance.
- Assuming that glucose’s chemical bonding nature is solely determined by the electronegativity of its constituent atoms.
FAQs
-
What is the hybridization of the carbon atoms in glucose?
Glucose’s carbon atoms are sp3 hybridized, meaning they have four electron pairs arranged tetrahedrally. -
Why are the carbon-oxygen bonds in glucose not completely ionic?
Due to the intermediate electronegativity difference between carbon and oxygen, the electrons in the carbon-oxygen bonds are not completely transferred, resulting in polar covalent bonds. -
How many resonance structures does glucose have?
Glucose has three resonance structures due to the delocalization of the double bond between the carbon and oxygen atoms. -
What are the applications of understanding glucose’s bonding nature?
Comprehending glucose’s bonding nature has applications in energy production, pharmaceutical development, food science, and molecular biology.
Conclusion
While glucose’s bonding nature is not strictly ionic or covalent, it exhibits characteristics of both. The polar covalent bonds and resonance in glucose’s molecular structure give rise to its unique properties and play a vital role in its biological functions and potential applications.