Introduction
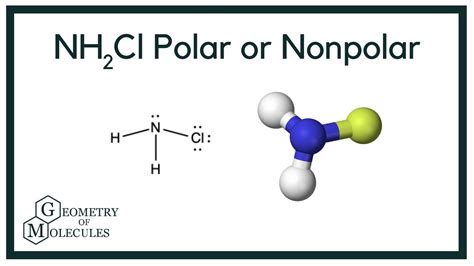
Chloramine (NH2Cl), a colorless and pungent gas with the systematic name monochloramine, has sparked considerable scientific interest due to its dual nature. Understanding its polarity is crucial for deciphering its behavior in various chemical processes. This article delves into the intricate molecular structure of NH2Cl, exploring its polarity and unraveling its implications in diverse applications.
Polarity: A Deeper Dive
Polarity refers to the uneven distribution of electrical charges within a molecule. Molecules with a permanent separation of partial positive and negative charges are deemed polar. In contrast, those with an equal distribution of charges are considered nonpolar.
The molecular geometry of NH2Cl plays a pivotal role in determining its polarity. It adopts a bent or V-shaped structure, deviating from linearity. The polarity of each chemical bond within the molecule contributes to the overall polarity of NH2Cl.
Electronegativity and Bond Polarity
Electronegativity, a measure of an atom’s ability to attract electrons, is a key factor in bond polarity. Chlorine (Cl) is more electronegative than nitrogen (N), and hydrogen (H) is less electronegative than both. This difference in electronegativity gives rise to polar covalent bonds within NH2Cl.
The N-H bond is slightly polar, with a partial negative charge on the nitrogen and a partial positive charge on the hydrogen. The N-Cl bond is more polar, with the chlorine atom acquiring a significant negative charge and the nitrogen atom carrying a positive charge.
Consequences of Polarity
The polarity of NH2Cl endows it with unique properties that influence its behavior in various physical and chemical processes.
Solubility
Polar molecules tend to dissolve in polar solvents, while nonpolar molecules dissolve better in nonpolar solvents. NH2Cl’s polarity enables it to dissolve in water, a polar solvent, to a great extent. This property is crucial for its role in water disinfection processes.
Reactivity
The polarity of NH2Cl affects its reactivity with other molecules. The partial positive charge on the nitrogen atom makes it susceptible to nucleophilic attack, while the partial negative charge on the chlorine atom attracts electrophiles. This reactivity is particularly important in the formation of chloramines in water treatment.
Applications of NH2Cl
The unique properties of NH2Cl have paved the way for its diverse applications in various fields.
Water Disinfection
NH2Cl is widely used as a disinfectant in water treatment facilities. Its polarity allows it to dissolve effectively in water and react with microorganisms, effectively eliminating pathogens.
Paper Industry
NH2Cl finds application in the paper industry as a bleaching agent. Its ability to react with lignin, a component of wood pulp, helps whiten paper and improve its quality.
Textile Industry
In the textile industry, NH2Cl is employed as a scouring agent to remove impurities and enhance the dyeability of fabrics.
Conclusion
NH2Cl is a polar molecule, as evidenced by its molecular geometry, electronegativity differences, and partial charge distribution. This polarity governs its solubility, reactivity, and applications in various fields, including water disinfection, paper manufacturing, and textile processing. Understanding the polarity of NH2Cl is essential for harnessing its full potential and developing novel applications in the future.
Additional Resources
[1] National Institute of Health: https://pubchem.ncbi.nlm.nih.gov/compound/Chloramine-T#section=Solubility
[2] Environmental Protection Agency: https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-chloramines
[3] American Water Works Association: https://www.awwa.org/Resources-Tools/Resources/Information-and-Resources-on-Disinfectants-and-Disinfection-Byproducts/Chloramines
[4] International Agency for Research on Cancer (IARC): https://www.iarc.who.int/wp-content/uploads/2018/06/IARC_Monographs_Vol101.pdf (pp. 45-82)