Sulfur is a non-metallic element that is essential for life. It is found in proteins, vitamins, and other biomolecules. Sulfur atoms have six valence electrons, which means that they can form six covalent bonds with other atoms.

Number of Unpaired Electrons
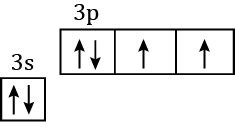
In a ground state sulfur atom, two of the valence electrons are unpaired. These unpaired electrons are responsible for the element’s chemical reactivity. Sulfur can easily form bonds with other atoms because it has two unpaired electrons that can be shared.
Electron Configuration
The electron configuration of a sulfur atom is 1s22s22p63s23p4. This means that the atom has two electrons in the first energy level, two electrons in the second energy level, and four electrons in the third energy level. The four electrons in the third energy level are arranged in three p-orbitals, each of which contains two electrons.
Chemical Reactivity
The two unpaired electrons in a sulfur atom make it a very reactive element. Sulfur can easily form bonds with other atoms, including hydrogen, oxygen, carbon, and nitrogen. Sulfur is also a good oxidizing agent, which means that it can donate electrons to other atoms or molecules.
Applications of Sulfur
Sulfur is used in a wide variety of applications, including:
- Fertilizers: Sulfur is an essential nutrient for plants, and it is often added to fertilizers to help promote plant growth.
- Batteries: Sulfur is used in the production of lead-acid batteries, which are commonly used in cars and other vehicles.
- Papermaking: Sulfur is used in the production of paper, where it helps to bleach the paper and improve its strength.
- Pharmaceuticals: Sulfur is used in the production of a variety of pharmaceuticals, including antibiotics, anti-inflammatory drugs, and laxatives.
Conclusion
Sulfur is a versatile element that is used in a wide variety of applications. The two unpaired electrons in a sulfur atom make it a very reactive element, which is responsible for its chemical properties and its usefulness in a variety of industrial and consumer products.
Additional Information
- The atomic number of sulfur is 16.
- The atomic weight of sulfur is 32.066.
- Sulfur is the tenth most abundant element in the universe.
- Sulfur is a solid at room temperature.
- Sulfur is yellow in color.
- Sulfur is insoluble in water.
- Sulfur is a good conductor of electricity.
- Sulfur is a semiconductor.
- Sulfur is a non-metal.
- Sulfur is a reactive element.
- Sulfur is a flammable element.
- Sulfur is a toxic element.